Structure of atom class 11 pdf
Structure of the Atom : Chapter Notes. Matter is made up of tiny particles called atoms. Atoms are further made of three fundamental particles or sub – atomic particles called electron, proton and neutron. Earlier Dalton postulated that atom is indivisible i.e. cannot be further divided which proved to be wrong by discovery of sub – atomic particles. Types of Sub – atomic Particles. Note
NCERT Solutions for Class 11 Chemistry Chapter 2 Structure Of Atom PDF Free Download of CBSE Board.
Vagupu has some of the best online Chemistry tutors for Class 11. Get your first free session with the top tutors today. If you are looking for any information in …
CBSE Test Papers class 11 Chemistry Structure of Atom. CBSE chapter wise practice papers with solution for class 11 Chemistry chapter 2 Structure of Atomfor free download in PDF format. 11th Chemistry chapter 2 Structure of Atom have many topics.
STRUCTURE OF ATOM. Atoms: Atom is the smallest indivisible particle of the matter. Atom is made of electron, proton and neutrons. PARTICLE ry
Chemistry Class 11 Important Questions are very helpful to score high marks in board exams. Here we have covered Important Questions on Structure of Atom for Class 11 Chemistry subject. Chemistry Important Questions Class 11 are given below.
An important feature of Thomson model of an atom was that mass of atom is considered to be evenly spread over the atom. 14.Thomson model of atom is also called as Plum pudding, raisin pudding or
Subject: ICT Based Support to Chemistry Textbook for Class XI . Some online resources like websites, videos and animations have been identified and integrated with the unit, 2, Structure of Atom for class XI chemistry textbook.
Structure of Atom class 11 notes chapter 2 chemistry Structure of Atom (Atomic Structure) Part 2 BOHR’S ATOMIC MODEL Bohr
25/04/2017 · In this video we cover all concepts of the chapter ‘Structure of Atom’ within one hour. Email me at aceitbiz@gmail.com if you have any questions.
Structure of Atom Multiple Choice Questions and Answers 1 PDF Download. Learn structure of atom multiple choice questions, O level chemistry online test 1 for e-learning, free online courses test.
Chemistry Notes for Class 11 Download in pdf Structure of Atom. Here we have covered Important Questions on Structure of Atom for Class 11 Chemistry subject. Chemistry Important Answers to Multiple Choice Questions. Study Material for Atomic Spectra of Structure of Atom of Chemistry of Class XI of Uttar Pradesh Board. Watch video Questions and doubts asked by students on Atomic …
JEE Structure of Atom, Chapter Notes, Class 11, Chemistry (IIT-JEE & AIPMT) Summary and Exercise are very important for perfect preparation. You can see some Structure of Atom, Chapter Notes, Class 11, Chemistry (IIT-JEE & AIPMT) sample questions with examples at the bottom of this page. Complete Structure of Atom, Chapter Notes, Class 11, Chemistry (IIT-JEE & AIPMT) chapter …
Chapter 2 structure of atom class 11 SlideShare

11th Class Online Prepration Studyadda.com
Free PDF download of Class 11 Chemistry revision notes & short key-notes for Chapter 2 – Structure of Atom to score high marks in exams, prepared by expert Chemistry teachers from latest edition of CBSE(NCERT) books.
class xi chemistry notes structure of atom distributor of Essential Oils and Aromatic Chemicals. Berjé is a family-owned business that has been in
19/05/2014 · Tags – Free Question bank for class 11, free IIT question bank maths for class 11, class 11 question bank pdf, class 11 IIT maths question b… ExamPCM Disclaimer
“Atomic Structure -1” Defining the Atom the electron to be 1.76 x 1011 C/kg. This ratio is constant for all materials. Mass of the Electron 1916 – Robert Millikan determines the mass of the electron: 1/1840 the mass of a hydrogen atom; has one unit of negative charge The oil drop apparatus Mass of the electron is 9.11 x 10-28 g . Millikan’s Oil Drop Experiment Charged droplet can

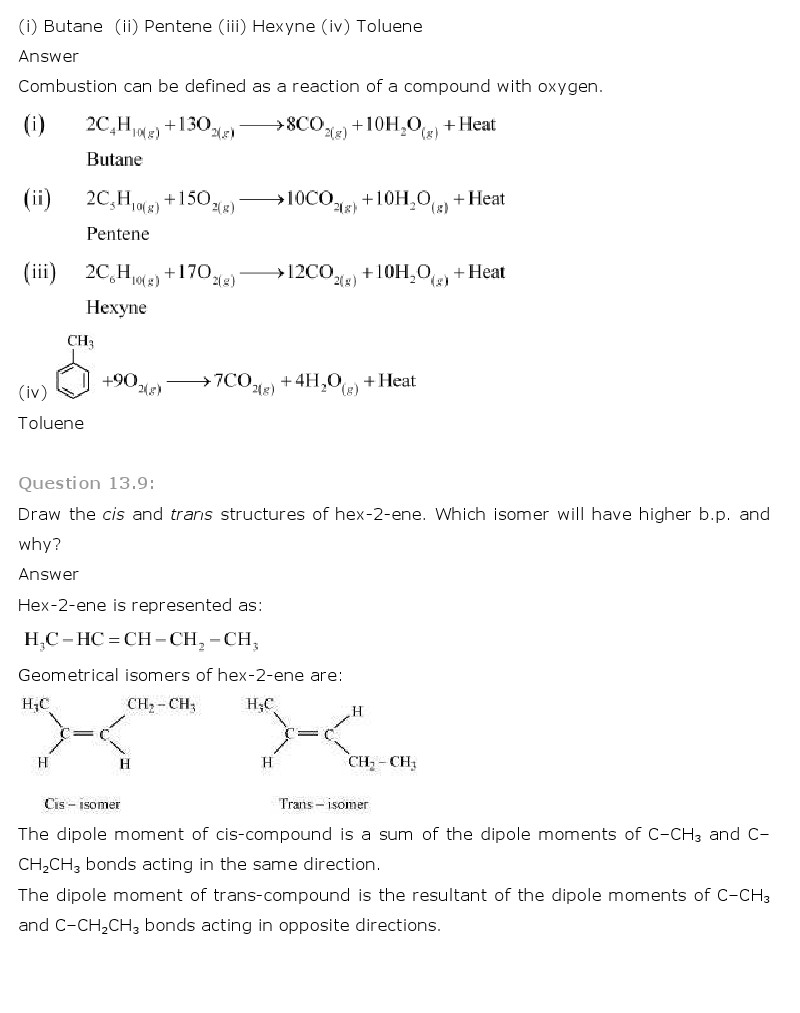
Q.3 Name the subatomic particles present in an atom. Atom is made up of smaller particles called subatomic particles. The sub-atomic particles are electrons, protons and neutrons.
Visit www.ncerthelp.com For All NCERT solutions, CBSE sample papers, Question papers, Notes for Class 6 to 12 CHAPTER 2 STRUCTURE OF ATOM • Atom …
Download as PDF, TXT or read online AIEEE Class XI Chem Structure of Atom. For Later. save. Related. Info. Embed. Share. Print. Search. Download. Jump to Page . You are on page 1 of 64. Search inside document . 000000000., + , + , + , + , + , + , + , + , + , + , + , + , + , + /111111111- AIEEE STRUCTURE OF ATOM C H E M I S T R Y S T U D Y M A T E R I A L NARAYANA INSTITUTE OF
The chemistry textbooks of class XI deal with structure of atom in a historical manner discussing Thomson, Rutherford, Bohr and Quantum Mechanical model. This paper is an effort to analyze one of the chapters; ‘Structure of Atom’,
For important questions on structure of atoms and other chapters of Class 11 chemistry, you can refer this link – CBSE Class 11 Chemistry- Chapter Wise Important Questions Free Download For other subjects of Class 11 and their important questions on each chapter, refer this link – CBSE Class 11 All Subjects Chapter Wise Important Questions
Structure of Atom (i) Calculate the number of electrons which will together weight one gram. (ii) Calculate the mass and charge of one mole of electrons.
Get Structure of Atom , Chemistry Chapter Notes, Video Lessons, Practice Test and more for CBSE Board Class 11 science only at TopperLearning. Get Structure of Atom , Chemistry Chapter Notes, Video Lessons, Practice Test and more for CBSE Board Class 11 science only at TopperLearning.
Free NCERT Solutions for Class 11 Chemistry solved by expert teachers from latest edition books and as per NCERT (CBSE) guidelines.Class 11 Chemistry NCERT Solutions and Extra Questions with Solutions to help you to revise complete Syllabus and Score More marks.

19/05/2012 · Atom has a tiny dense central core or the nucleus which contains practically the entire mass of the atom leaving the rest of the atom almost empty. 2. The entire positive charge of the atom is located on the nucleus.
2.1. (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. 2.2. (i) Calculate the total number of electrons present in one mole of methane. (ii) Find (a) the total number and (b) the total mass of neutrons in 7 mg of 14
CBSE Class 11 Chemistry – Basic Concepts and Structure Of Atom. Chapter wise assignments are being given by teachers to students to make them understand the chapter concepts.
The wave number of the spectral line in the emission spectrum of hydrogen will equal to times the Rydberg’s constant if the electron jumps from _____.
Postulate: -Atom is a sphere of positive charge in which number of electrons are embedded. Limitat ions: – Could not satisfactorily explain the results of scattering experiment carried out by Rutherford.
Chemistry Notes Class 11 PDF Chemistry Notes For Class 11 PDF Chemistry comprises compounds composed of a combination of atoms, behavior, their composition, their structure as well as different reactions due to imbalance nature of molecules and atoms.
NCERT Solutions for Class 11 Chemistry Learn CBSE
These NCERT chemistry solutions class 11 include a detailed explanation of all the important concepts like Structure of Atom, Periodicity, Chemical Bonding, Thermodynamics, Equilibrium, Redox Reaction, Hydrogen, S Block and P Block Elements, Organic Chemistry, Hydrocarbons, Environmental Chemistry. All these NCERT class 11 chemistry solutions are prepared by the experts in their respective
Atomic structure An atom consists of a nucleus composed of protons and neutrons and electrons which encircle the nucleus. Protons and electrons have same and opposite charge of
Find the atomic structure pdf here. The study of the atom and its structure has paved the way for numerous inventions that have played a significant role in the development of humankind. To follow more download Byju’s-the learning app.
Atom. John Dalton proposed (in 1808) that atom is the smallest indivisible particle of matter. Atomic radii are of the order of 10-8 cm. It contain three subatomic particles namely electrons, protons and …
CHEM 462 Prerequisite material To remind you of some things that you should have seen in earlier courses, I have posted 6 files, prereq#.pdf (# – 1–6), in the ‘Handouts’ section of the
Vedantu.com – No.1 online tutoring company in India provides you Free PDF download of NCERT Solutions for Class 11 Chemistry Chapter 2 – Structure of Atom solved by Expert Teachers as per NCERT (CBSE) Book guidelines.
considers the structure of an atom, its size should also be considered. It was already known that an approximation for the volume of an atom could be estimated by dividing the volume of 1 mol of solid by Avogadro’s constant. Sample Exercise 2.3 The volume of one molecule of water Assuming that the water molecule is a cube, calculate the length of an edge of this cube. Using the value – indesign images blurry pdf interactive 3 Address: Plot No 420, Behind Shopprix Mall, Vaishali Sector 5, Ghaziabad – 201010 M: 9999907099, 9818932244 O: 0120-4130999 Website: www.vaishalieducationpoint
Important Class Of Biological Structure Of Nucleotide. 1’ C. 2’ PPT. Presentation Summary : Important class of biological Structure of Nucleotide. 1’ C. 2’ C. 3 between sugar and another organic molecule by way of intervening N or O atom.
Atomic Structure MODULE – 2 Notes Atomic Structure and Chemical Bonding hemistry has been defined as the study of matter in terms of its structure, composition and the properties. As you are aware, matter is made up of atoms, and therefore an understanding of the structure of atom is very important. You have studied in your earlier classes that the earliest concept of atom ( smallest
The electronic configuration of sodium atom (a tomic number 11) is 2,8,1. Since it is highly electropositive, it readily loses an electron to attain the stable configuration of the nearest noble gas (ne on) atom. It becomes a positively charged sodium cation (Na+) in the process. 80 MODULE – 2 Chemistry Notes Atomic Structure and Chemical Bonding Na Na+ + e–; H = 493.8 kJ mol–1 2,8,1 …
STRUCTURE OF ATOM Class 11 Chemistry CBSE Board Free download Aoms: Atom is the smallest indivisible particle of the matter. Atom is made of electron, proton and neutrons.
6 Chapter 2 11 Electronegativity The electronegativity of the elements, adapted from Smith&Hashemi Electronegativity is a degree to which an atom attracts electron to itself
11th Class Online Coaching The number of package may vary time to time. Study packages are available in soft (PDF format) copy only. After buying you will be able to download them, and then read over any device (Mobile, tab, desktop, laptop etc.) having PDF …
19/05/2012 · About the structure of atom a theory was put on by John Dalton in 1808. According to this theory matter was made from small indivisible particles called atoms. According to this theory matter was made from small indivisible particles called atoms.
9/7/2017 Chemistry Notes for Class 11 STRUCTURE OF ATOM Download in pdf 1/7 NCERT Solutions, CBSE Sample paper, Latest Syllabus, NCERT Books, Last Year Question Papers and Many More…
Download CBSE Revision Notes for CBSE Class 11 Chemistry Structure of Atom Discovery of Electron, Proton and Neutron, atomic number, isotopes and isobars.
20/08/2016 · 67 videos Play all Structure of Atom – Chemistry Class 11 CBSE Chemistry Class XI Mole Concept Tips and Tricks – Duration: 14:32. PLAY Chemistry 836,990 views
Find PowerPoint Presentations and Slides using the power of XPowerPoint.com, find free presentations research about Structure Of Atom Class 11 PPT Sponsored Links Displaying structure of atom class 11 PowerPoint Presentations
The following section consists of Chemistry Multiple Choice questions on Atomic Structure For competitions and exams. Select the correct option to test your skills on Atomic Structure. Select the correct option to test your skills on Atomic Structure.
Class IX – Structure of the AtomNCERT –Physics The total number of electrons in a carbon atom is 6. The distribution of electrons in carbon atom is given by: First orbit or K-shell = 2 electrons Second orbit or L-shell = 4 electrons Or, we can write the distribution of electrons in a carbon atom as 2, 4. The total number of electrons in a sodium atom is 11. The distribution of electrons in
Atom or a group of atoms which possess any ‘free valency’ are called as Groups. If their are two structure of same molecular formula then some prefix (n, iso, neo) are used two differentiate them.
5/02/2015 · 1 year n0tes chemistry new st CHAPTER 5 ATOMIC STRUCTURE MCQs Q.1 Splitting of spectral lines when atoms are subjected to strong electric field is called
in class XII. These results suggested the particulate nature of electricity. An insight into the structure of atom was obtained from the experiments on electrical discharge through gases. Before we discuss these results we need to keep in mind a basic rule regarding the behaviour of charged particles : “Like charges repel each other and unlike charges attract each other”. In mid 1850s many
Solved Questions of Atomic Structure askIITians
Therefore, λ for the hydrogen atom would be 16 times greater than λ for oxygen atom. Problem 11: A 1 MeV proton is sent against a gold leaf (Z = 79). Calculate the distance of …
ch-2 structure of atom class 11: File Size: 686 kb: File Type: pdf: Download File. ch-3 classification of elements class 11 : File Size: 312 kb: File Type: pdf: Download File. ch4 chemical bonding and molecular structure class_11: File Size: 1111 kb: File Type: pdf: Download File. ch-5 states of matter class 11: File Size: 336 kb: File Type: pdf: Download File. ch-6 Thermodynamics class 11
CLASS – XI CHEMISTRY (Structure of Atom) Topic:- Orbital’s and Quantum Numbers 1. Which orbital is non – directional? [1] 2. What is the meaning of quantization of energy? [1] 3. Why is energy of 1s electron lower than 2s electron? [1] 4. Which quantum number determines (i) energy of electron (ii) Orientation of orbitals. [2] 5. What is nodal surface or nodes? [1] 6. How many spherical
Structure of Atom Class 11 CBSE (Hindi) YouTube
Structure Of Atom For Class 11th PPT Xpowerpoint
CBSE Class 11 Chemistry MCQs – Structure of Atom CBSE and NCERT students can refer to the attached file. All educational material on the website has been prepared by the best teachers having more than 20 years of teaching experience in various schools.
Class 11 Important Questions – Structure of Atom Summary and Exercise are very important for perfect preparation. You can see some Important Questions – Structure of Atom sample questions with examples at the bottom of this page. Complete Important Questions – Structure of Atom chapter (including extra questions, long questions, short questions, mcq) can be found on EduRev, you can …
Chapter 2 structure of atom class 11 1. Structure of Atom 1 GRADE 11 2. DISCOVERY OF AN ELECTRON An electron was discovered by cathode ray discharge tubes experiment. A cathode ray tube is made of glass containing two thin pieces of metal called electrodes, sealed in it.
Free Download NCERT Solutions for Class 11 Chemistry in PDF form for UP board (intermediate students) and CBSE board from 2018-2019 onward. Assignments, notes, Revision books and notes, solved questions with answers in PDF format. UP board also using NCERT textbooks following the same CBSE syllabus 2018-19.
CBSE Class 11 – Chemistry – Structure of Atom – CBSE

Structure of Atom Multiple Choice Questions Answers
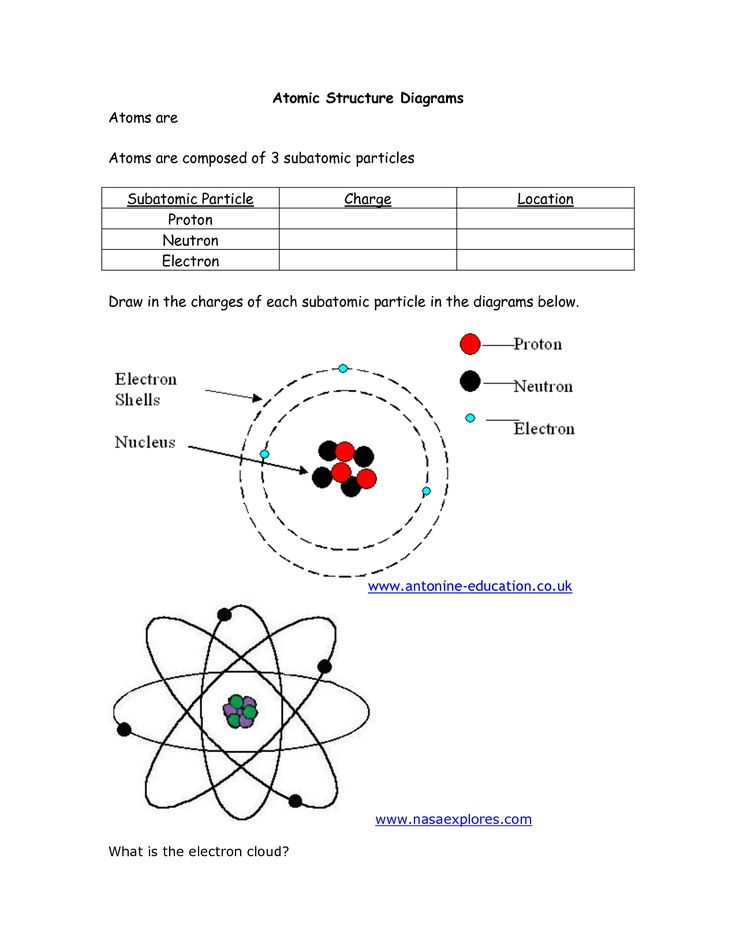
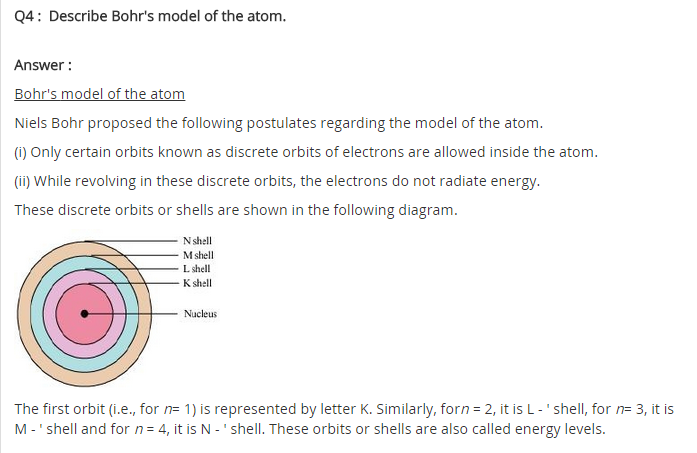
Home » Topics » Class 11 » Chemistry » Structure Of Atom » Bohr’s Model of an atom. Bohr’s Model of an atom. February 10, 2017 By Mrs Shilpi Nagpal 1 Comment Neils Bohr, a Danish physicist in 1913 proposed a new model of atom. This new model is called Bohr’s Model of atom. Postulates of Bohr’s model of an atom . 1)An atom consists of a small, heavy positively charged nucleus in
Structure of Atom E-worksheet

What are the important questions of the chapter on the
https://en.m.wikipedia.org/wiki/Functional_group
Chemistry MCQ on Atomic Structure Examtime Quiz
– structure of atom class 11 cbse pdf EDUBUZZ NOTES
NCERT Solutions for Class 11 Chemistry in PDF for 2018-19

Structure of Atom Atomic Structure MCQs Question
Structure of the Atom Chapter Notes – DronStudy.com
CBSE Class 11 Chemistry- Chapter Wise Important Questions
Bohr’s Model of an atom Chemistry Class 11 Structure
25/04/2017 · In this video we cover all concepts of the chapter ‘Structure of Atom’ within one hour. Email me at aceitbiz@gmail.com if you have any questions.
6 Chapter 2 11 Electronegativity The electronegativity of the elements, adapted from Smith&Hashemi Electronegativity is a degree to which an atom attracts electron to itself
Vagupu has some of the best online Chemistry tutors for Class 11. Get your first free session with the top tutors today. If you are looking for any information in …
ch-2 structure of atom class 11: File Size: 686 kb: File Type: pdf: Download File. ch-3 classification of elements class 11 : File Size: 312 kb: File Type: pdf: Download File. ch4 chemical bonding and molecular structure class_11: File Size: 1111 kb: File Type: pdf: Download File. ch-5 states of matter class 11: File Size: 336 kb: File Type: pdf: Download File. ch-6 Thermodynamics class 11
CBSE Test Papers class 11 Chemistry Structure of Atom. CBSE chapter wise practice papers with solution for class 11 Chemistry chapter 2 Structure of Atomfor free download in PDF format. 11th Chemistry chapter 2 Structure of Atom have many topics.
Structure of Atom (i) Calculate the number of electrons which will together weight one gram. (ii) Calculate the mass and charge of one mole of electrons.
Class Xi Chemistry Notes Structure Of Atom careertest.in
Bohr’s Model of an atom Chemistry Class 11 Structure
Find the atomic structure pdf here. The study of the atom and its structure has paved the way for numerous inventions that have played a significant role in the development of humankind. To follow more download Byju’s-the learning app.
An important feature of Thomson model of an atom was that mass of atom is considered to be evenly spread over the atom. 14.Thomson model of atom is also called as Plum pudding, raisin pudding or
11th Class Online Coaching The number of package may vary time to time. Study packages are available in soft (PDF format) copy only. After buying you will be able to download them, and then read over any device (Mobile, tab, desktop, laptop etc.) having PDF …
Structure of Atom (i) Calculate the number of electrons which will together weight one gram. (ii) Calculate the mass and charge of one mole of electrons.
Chapter 2 structure of atom class 11 1. Structure of Atom 1 GRADE 11 2. DISCOVERY OF AN ELECTRON An electron was discovered by cathode ray discharge tubes experiment. A cathode ray tube is made of glass containing two thin pieces of metal called electrodes, sealed in it.
Bohr’s Model of an atom Chemistry Class 11 Structure
Chemistry Class 11 Important Questions are very helpful to score high marks in board exams. Here we have covered Important Questions on Structure of Atom for Class 11 Chemistry subject. Chemistry Important Questions Class 11 are given below.
Bohr’s Model of an atom Chemistry Class 11 Structure
Class 11 Chemistry Revision Notes for Chapter 2