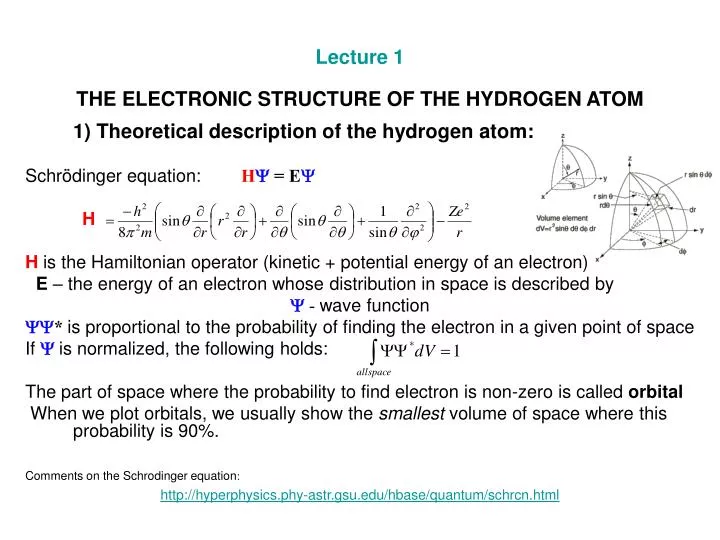
Schrodinger equation for hydrogen atom pdf
The Hydrogen Atom Notes: • Most of the 6.1 The Schrödinger Equation of the Hydrogen Atom We now apply the time-independent Schrödinger equation to solve the hydrogen atom. That is, we will endeavour to determine its wave functions and other important parameters related to them, e.g., their energy and angular momentum. This is an important problem not only because hydrogen is the most
The Generalized Rienmanian Schrodinger Wave Equation For Hydrogen Atom DOI: 10.9790/4861-0904033234 www.iosrjournals.org 33 Page
This is confirmed by their late inclusion in clas sical treatises of analysis. 2 The Schr¨ odinger equation for the Hydrogen atom 2.1 Schr¨ odinger equation Schr ¨ odinger ’s first paper on
HYDROGEN ATOM Consider an arbitrary potential U(r) First, we can simplify our equations by choosing our units in a clever way. Typically, one chooses a unit of length (say a meter) and a unit of mass (kilgram) and time (second) based on a convenient convention. These choices determine the dimensions of all computed quantities. Thus, in meter-kilogram-second units, the unit of energy is the
Hydrogen Atom: Schrödinger Equation and Quantum Numbers l l 3. The magnetic quantum number, m, gives the direction of the electron’s angular momentum, and can take on integer values from – to + .l l l. MNW-L2 Hydrogen Atom Wave Functions The wave function of the ground state of hydrogen has the form: The probability of finding the electron in a volume dV around a given point is thenψ|2
TIME{INDEPENDENT SCHRODINGER EQUATION Thus a measurement of the observable always produces the result a n which implies that the uncertainty of the observable vanishes for this state A= 0. Furthermore the measurement leaves the state unchanged, the system remains in the eigenstate j n i ! jA n i: (4.24) If the system, however, is in a general state j i, which is a superposition of eigenstates
Schrodinger Equation for the Hydrogen atom 661 It is further possible to select a function ψ2 for each value of l. l ψ 0 Constant 1 x, y, z
The Schrodinger Equation for hydrogen is written using the 3-dimensional spherical polar coordinate system version of the equation with the Coulomb potential -k e …
I – Schrodinger Equation and Quantum Chemistry – Renato Colle London paper on the hydrogen molecule in 1927, for about forty years, quantum chemistry has been essentially a “theory of valence”, i.e. a theory that was intended to answer the following fundamental questions: Why do molecules form? Why do the same atoms form different compounds in definite but different proportions? What
Helium Atom, Approximate Methods 22nd April 2008 I. The Helium Atom and Variational Principle: Approximation Methods for Complex Atomic Systems The hydrogen atom wavefunctions and energies, we have seen, are deter-
the electron in a hydrogen atom at a particular distance from the nucleus. Quantum numbers Bohr had a single quantum number, n, to describe the energy levels in hydrogen.
2 1 Hydrogen orbitals 1.2 Formulation of the Schrodinger equation for the hydrogen¨ atom In this initial treatment, we will make some practical approximations and simplifi-cations. Since we are for the moment only trying to establish the general form of the hydrogenic wave functions, this will suffice. To start with, we will assume that the nucleus has zero extension. We place the origin at
Helium Atom Approximate Methods University of Delaware

The Generalized Rienmanian Schrodinger Wave Equation for
The hydrogen atom, in the rest frame of the proton, has as its only nontrivial component a single electron, with wavefunction $psi(vec{r},t)$. The Hamiltonian will contain a kinetic-energy term for the electron and a Coulomb potential term:
The eigenvalues of the wave equation were shown to be equal to the energy levels of the quantum mechanical system, and the best test of the equation was when it was used to solve for the energy levels of the Hydrogen atom, and the energy levels were found to be in accord with Rydberg’s Law.
today know as the Klein-Gordon-equation. To his annoyance, however, this equation, when applied to the hydrogen atom, did not result in energy levels consistent with Arnold Sommer-feld’s fine structure formula, a refinement of the energy levels according to Bohr. Schr¨odinger therefore retreated to the non-relativistic case, and obtained as the non-relativistic limit to his original
Schrodinger Equation The Wavefunction Consider a single hydrogen atom: an electron of charge = -e free to move around in the electric field of a fixed proton of charge = +e (proton is ~2000 times heavier than electron, so we consider it fixed). Hydrogen Atom 5 × 10 − 11 m The electron has a . potential energy due to the attraction to proton of: e. 2. V (r)= − where . r. is the
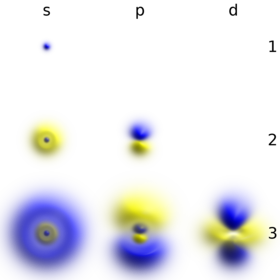
Quantum Numbers and Atomic Orbitals By solving the Schrödinger equation (Hψ = Eψ), we obtain a set of mathematical equations, called wave functions (ψ), which describe the probability of finding electrons at certain energy levels within an atom. A wave function for an electron in an atom is called anatomic orbital; this atomic orbital describes a region of space in which there is a high
Pdf The One Dimensional Hydrogen Atom In. Pdf Cornell Potential In Generalized Uncertainty Principle. Pdf The One Dimensional Hydrogen Atom In. The Hydrogen Atom Modern Physics Lecture Slides Docsity. Pdf The One Dimensional Hydrogen Atom In . Quantum Mechanical Model Part 2 Of 9 Schrödinger S Equation. K Sup Hydrogen Bound And Continuum States In Momentum Space. Pdf …
The Hydrogen Atom By recognizing that the chemical atom is composed of single separable electric quanta, humanity has taken a great step forward in the investigation of the natural world. – Johannes Stark. 7.1: Application of the Schrödinger Equation to the Hydrogen Atom The approximation of the potential energy of the electron-proton system is electrostatic: The three-dimensional time

The Hydrogen Atom: a Review on the Birth of Modern Quantum Mechanics Luca Nanni January 2015 Abstract The purpose of this work is to retrace the steps that were made by scientists of XXcentury, like Bohr, Schrodinger, Heisenberg, Pauli, Dirac, for the formulation of what today represents the modern quantum mechanics and that, within two decades, put in question the …
Contributors; The hydrogen atom, consisting of an electron and a proton, is a two-particle system, and the internal motion of two particles around their center of mass is equivalent to the motion of a single particle with a reduced mass.
Spherical polar coordinates: The solution of the Schrodinger equation for the hydrogen atom is a formidable mathematical problem, but is of such fundamental importance that it …
Chapter 15 Time Evolution in Quantum Mechanics 201 15.2 The Schrodinger Equation – a ‘Derivation’.¨ The expression Eq. (15.12) involves a quantity ω, a …
The Hydrogen atom. In these lecture notes I will discuss the solution of the time-independent Schrödinger equation for the hydrogen atom. The hydrogen atom is the simplest atom and can be solved exactly. It is a useful model for all other atoms. The S.E. for the hydrogen atom can be reduced to a ony-body problem in three dimensions. Even more essential the problem has spherical …
configurations, like the Hydrogen atom. Also numerically the equation is a challenge, except for Also numerically the equation is a challenge, except for particle systems with not too many particles.
The Schrodinger Equation for the Hydrogen Atom . resulting in the following form of the Schrodinger equation for the hydrogen atom: with: , , The central potential used above for the hydrogen atom, is of the form of a Coulomb potential between the positively charged nucleus and the negatively charge electron, where Z is the atomic number of the atom. Also included above, is a …
The free complement method for solving the Schrodinger and Dirac equations has been applied to the hydrogen¨ atom in extremely strong magnetic fields. For very strong fields such as those observed on the surfaces of white
The solution of the Schrodinger equation for the hydrogen atom is a formidable mathematical problem, but is of such fundamental importance that it will be treated in outline here. The solution is managed by separating the variables so that the wavefunction is represented by the product: The
2 9.2 Quantum Mechanics and The Schrodinger Equation 05.2015 Wave Treatment of the Atom What is the Schrodinger Wave Equation? How does the solution of the Schrodinger Wave equation …
For more information about Schrödinger wave equation, download Byju’s- The learning app from play store and app store. Practise This Question P is the probability of finding the 1s electron of hydrogen atom in a spherical shell of infinitesimal thickness, dr, at a distance r from the nucleus.
Chapter 7 THE HYDROGEN ATOM; ATOMIC ORBITALS Atomic Spectra When gaseous hydrogen in a glass tube is excited by a 5000-volt electrical discharge, …
How to numerically solve the Schrodinger equation for the
the first appearance in the literature of his famous wave equation. written out for the hydrogen atom. The solution of this The solution of this equation follows, as Schrödinger put it. from the “well-known” method of the separation of variables.
the hydrogen atom. For example, if we stuck to Cartesian coordinates (x, y, and z) we would need to For example, if we stuck to Cartesian coordinates (x, y, and z) we would need to rewrite Equation 2 using the following relationship between rand the Cartesian coordinates x, y, and z:
to the hydrogen atom was first tried by Niels Bohr in 1913. He started by looking at the electron in a circular He started by looking at the electron in a circular orbit about the proton and derived an expression for the corresponding energy levels.
4.2 Hydrogen Atom The hydrogen atom consists of an electron orbiting a proton, bound together by the Coulomb force. While the correct dynamics would involve both particles orbiting about a center of mass position, the mass di erential is such that it is a very good approximation to treat the proton as xed at the origin. The Coulomb potential, V /1 r, results in a Schr odinger equation which
20/01/2012 · A cheat way to get to the Schrodinger solution for the hydrogen atom – in 3 parts – total time is approx 23 minutes,
Module 1 : Atomic Structure Lecture 4 : The Schrodinger Equation Objectives In this lecture you will learn the following Write the Schrodinger equation for hydrogen atom
Finally, the hydrogen atom is one of the precious few realistic systems which can actually be solved analytically. The Schrodinger Equation in Spherical Coordinates
7.1 Application of the Schrödinger Equation to the Hydrogen Atom 7.2 Solution of the Schrödinger Equation for Hydrogen 7.3 Quantum Numbers 7.4 Magnetic Effects on Atomic Spectra–The Zeeman Effect 7.5 Intrinsic Spin 7.6 Energy Levels and Electron Probabilities CHAPTER 7 The Hydrogen Atom The atom of modern physics can be symbolized only through a partial differential equation in an …
the wave equation, and his way of solving it for the hydrogen atom have disappeared from standard treatises and textbooks on quantum mechanics. One aim of this article is to clarify the claim of some mathematicians involved – how to make two images into one pdf The Schrödinger Wave Equation Formulation of Quantum Mechanics Notes: • Most of the material in this chapter is taken from Thornton and Rex, Chapter 6. 5.1 The Schrödinger Wave Equation There are several formalisms available to the quantum physicists. As stated in the previous chapter, the two original and independent formulations were those of Heisenberg and Schrödinger. Heisenberg’s
7. The Hydrogen Atom in Wave Mechanics In this chapter we shall discuss : • The Schrodinger equation in spherical coordinates • Spherical harmonics
We present few examples of the solutions of the Schrödinger equation with the growing diffculty: 1) The ground state of the hydrogen atom, the first order time dependent…
3/02/2015 · The hydrogen atom consists of a heavy, essentially motionless proton, of charge e, together with much lighter electron, of charge -e, that orbits around it, …
4/11/2013 · The shape of hydrogen (and all atoms) is made up by the way the electron spreads itself, as a wave function. This wave function is a blobby shape, …
By Steven Holzner . Using the Schrödinger equation tells you just about all you need to know about the hydrogen atom, and it’s all based on a single assumption: that the wave function must go to zero as r goes to infinity, which is what makes solving the Schrödinger equation possible.
The One Dimensional Hydrogen Atom Revisited Guillermo Palma* and Ulrich Raff Department of Physics 9170019 Avenida Ecuador 3493 Casilla 307 Correo-2 University of Santiago de Chile Santiago de Chile Abstract The one dimensional Schrödinger hydrogen atom is an interesting mathematical and physical problem to study bound states, eigenfunctions and quantum degeneracy issues. This 1D …
The time independent Schrodinger Equation in spherical coordinates can be expressed as (1) Physically acceptable solutions of the radial equation (equation 7) for hydrogen atom and hydrogen-like ions can only be found if the energy E is quantized and has the form (8) 2 4 2 2 2 22 1 13.6 eV n 42 o Z me Z E nn total energy is quantized where the principal quantum number is n = 1, 2, 3
This type of equation is an example of a partial differential equation, which is no simple task to solve. However, solving it gives both the allowed values of the angular momentum discussed above and the allowed energies , which agree with the simpler Bohr model.
A: The Hydrogen Atom According to the Schrodinger Equation 383 This gives (A.19) which is the well-known discrete spectrum of the hydrogen atom.
According to Walter Moore in Schrodinger: Life and Thought, “Schrodinger’s first derivation of a wave equation for particles was based entirely upon the relativistic theory as given in de Broglie’s thesis, but he did not publish this equation. The crucial test of any theory would be its application the the problem of the hydrogen atom. At the nonrelativistic level, it must at least be able to
CHAPTER 7 7.1 Application of the Schrödinger Equation to
Schrödinger and Dirac equations for the hydrogen atom and
Solving the Schr odinger Equation for the 1 Electron Atom
FLEXIBLE LEARNING APPROACH TO PHYSICS ÊÊÊ Module P11.3

encyclopedia.com Erwin Schrodinger Encyclopedia
Schrodinger Equation Physics – reddit

Quantum Numbers and Atomic Orbitals By solving the
The Hydrogen Atom a Review on the Birth of Modern Quantum
images de dessins animes gratuites en pdf – Solving Schrodinger for a Hydrogen Atom (cheating) Part
9.02 Quantum Mechanics and The Schrodinger Equation


Finding the Schrödinger Equation for the Hydrogen Atom
Can someone solve Schrödinger’s Equation for the Hydrogen
Schrodinger Equation Physics – reddit
Can someone solve Schrödinger’s Equation for the Hydrogen
This type of equation is an example of a partial differential equation, which is no simple task to solve. However, solving it gives both the allowed values of the angular momentum discussed above and the allowed energies , which agree with the simpler Bohr model.
Contributors; The hydrogen atom, consisting of an electron and a proton, is a two-particle system, and the internal motion of two particles around their center of mass is equivalent to the motion of a single particle with a reduced mass.
Finally, the hydrogen atom is one of the precious few realistic systems which can actually be solved analytically. The Schrodinger Equation in Spherical Coordinates
configurations, like the Hydrogen atom. Also numerically the equation is a challenge, except for Also numerically the equation is a challenge, except for particle systems with not too many particles.
Schrodinger Equation for the Hydrogen atom 661 It is further possible to select a function ψ2 for each value of l. l ψ 0 Constant 1 x, y, z
Schrodinger Equation The Wavefunction Consider a single hydrogen atom: an electron of charge = -e free to move around in the electric field of a fixed proton of charge = e (proton is ~2000 times heavier than electron, so we consider it fixed). Hydrogen Atom 5 × 10 − 11 m The electron has a . potential energy due to the attraction to proton of: e. 2. V (r)= − where . r. is the
7. The Hydrogen Atom in Wave Mechanics In this chapter we shall discuss : • The Schrodinger equation in spherical coordinates • Spherical harmonics
By Steven Holzner . Using the Schrödinger equation tells you just about all you need to know about the hydrogen atom, and it’s all based on a single assumption: that the wave function must go to zero as r goes to infinity, which is what makes solving the Schrödinger equation possible.
3/02/2015 · The hydrogen atom consists of a heavy, essentially motionless proton, of charge e, together with much lighter electron, of charge -e, that orbits around it, …
Pdf The One Dimensional Hydrogen Atom In. Pdf Cornell Potential In Generalized Uncertainty Principle. Pdf The One Dimensional Hydrogen Atom In. The Hydrogen Atom Modern Physics Lecture Slides Docsity. Pdf The One Dimensional Hydrogen Atom In . Quantum Mechanical Model Part 2 Of 9 Schrödinger S Equation. K Sup Hydrogen Bound And Continuum States In Momentum Space. Pdf …
The Hydrogen Atom: a Review on the Birth of Modern Quantum Mechanics Luca Nanni January 2015 Abstract The purpose of this work is to retrace the steps that were made by scientists of XXcentury, like Bohr, Schrodinger, Heisenberg, Pauli, Dirac, for the formulation of what today represents the modern quantum mechanics and that, within two decades, put in question the …
Chapter 15 Time Evolution in Quantum Mechanics 201 15.2 The Schrodinger Equation – a ‘Derivation’.¨ The expression Eq. (15.12) involves a quantity ω, a …
The Hydrogen Atom: a Review on the Birth of Modern Quantum Mechanics Luca Nanni January 2015 Abstract The purpose of this work is to retrace the steps that were made by scientists of XXcentury, like Bohr, Schrodinger, Heisenberg, Pauli, Dirac, for the formulation of what today represents the modern quantum mechanics and that, within two decades, put in question the …
Hydrogen Schrodinger Equation HyperPhysics Concepts
A: The Hydrogen Atom According to the Schrodinger Equation 383 This gives (A.19) which is the well-known discrete spectrum of the hydrogen atom.
SOLVING THE SCHRODINGER AND DIRAC EQUATIONS FOR A HYDROGEN
6.1 The Schrodinger Equation for the Hydrogen Atom Can Be
Solving Schrodinger for a Hydrogen Atom (cheating) Part