Quantum mechanical model of the atom pdf
Using our concept, we also mathematically derive the form of quantum mechanical wave function of free particle which is conventionally a postulate of quantum mechanics. Thus, we prove that the
CH40S Mr. Smith Historical Development of the Quantum-Mechanical Model Rutherford visualized the atom as a miniature solar system, with electrons circling the nucleus in
1 7.4 The Quantum-Mechanical Model of the Atom • Bohr’s model of the H atom – Assumes the quantization without explanation – Does not take into account Heisenberg’s
The Quantum Mechanical Model of the Atom were found to exhibit certain characteristics, including diffraction (the bending of waves around corners) and interference (the adding or subtracting of energies when waves overlap).
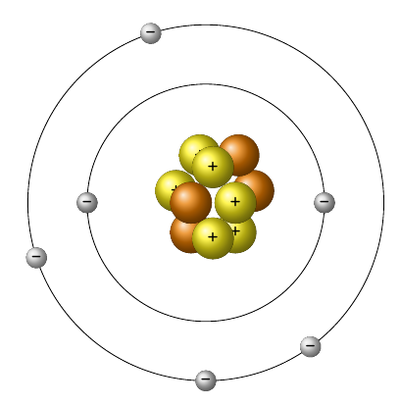
The Quantum Mechanical Atom Levels and Sublevels Images displayed in all types of media depict atoms as dense spherical nuclei surrounded by orbiting electrons.
The quantum scissors device for sending qubit states of light back in time. BS1 and BS2 are 50:50 beam splitters. The rectangles are light sources and the semi-ovals are photon detectors.
The Quantum Mechanical Model of the Hydrogen Atom (Reference: Section 7.4 (as is in the Bohr model of the hydrogen atom). Instead, the position of the electron can only be thought of in terms of the probability that an electron will be found within a certain distance of the nucleus. An atomic orbital is a region of space around a nucleus that one is likely to find an electron. The lowest
7HC the Quantum Mechanical Model – Download as Powerpoint Presentation (.ppt), PDF File (.pdf), Text File (.txt) or view presentation slides online. O Scribd é o …
160 Chapter 8 The Quantum Mechanical Atom Multiple Choice Section 8.1 3. What is the wavelength of electromagnetic radiation which has a frequency of 4.464 x 1014 s-1?
from a photon of light that that “hit” the atom, we call this event an absorption event. The photon’s energy was absorbed by the electron (destroying the photon), and the electron ends up in a higher energy level. Comparison of Bohr’s Model with the Quantum (or Wave) Mechanics Model Bohr’s Model Wave Mechanics Model (Schrödinger) e-‘s are in orbits e ‘s are “in” orbitals e-in an orbit is a
Energy: The Bohr model and the Quantum Mechanical model of the atom both assign specific energies to an electron. In the Bohr model the energy of an …
Bohr Model of the Atom (contd.)! • The Bohr Model led to the concept that energy is quantized: An atom cannot have a continuous range of energies (like stairs rather than a ramp).”
12/5/2014 1 The Quantum Mechanical View of the Atom Chemistry Timeline #1 B.C. 400 B.C. Democritus and Leucippus use the term “atomos” 1500’s
This is a test designed to help grade 12 University Chemistry students practice quantum mechanics before a big unit test. (Such as myself)
View Unit 2 The Quantum Mechanical Model of the Atom.pdf from CHEM 1120 at Langara College. UNIT 2 THE QUANTUM-MECHANICAL MODEL OF THE ATOM 1 A. The Schrdinger Equation 1. Quantum mechanics (wave
Quantum Mechanical Model of Atom EDUREV.IN

7.4 The Quantum-Mechanical Model of Atomic Orbitals the Atom
A quantum mechanical model of adaptive mutation Request PDF
– the hydrogen atom student guide answer key
The Quantum Mechanical View of the Atom PC|MAC
ALE 4. The Quantum Mechanical Model of the Hydrogen Atom
Quantum Mechanical Model of Atom edurev.in
The Quantum Mechanical Model of the Atom howechem.net
Comparison of Bohr’s Model with the Quantum (or Wave
https://en.wikipedia.org/wiki/Atomic_orbital_model
–
A quantum mechanical model of adaptive mutation Request PDF
Quantum Mechanical Model of Atom edurev.in
Bohr Model of the Atom (contd.)! • The Bohr Model led to the concept that energy is quantized: An atom cannot have a continuous range of energies (like stairs rather than a ramp).”
The Quantum Mechanical Model of the Hydrogen Atom (Reference: Section 7.4 (as is in the Bohr model of the hydrogen atom). Instead, the position of the electron can only be thought of in terms of the probability that an electron will be found within a certain distance of the nucleus. An atomic orbital is a region of space around a nucleus that one is likely to find an electron. The lowest
This is a test designed to help grade 12 University Chemistry students practice quantum mechanics before a big unit test. (Such as myself)
The Quantum Mechanical Atom Levels and Sublevels Images displayed in all types of media depict atoms as dense spherical nuclei surrounded by orbiting electrons.
160 Chapter 8 The Quantum Mechanical Atom Multiple Choice Section 8.1 3. What is the wavelength of electromagnetic radiation which has a frequency of 4.464 x 1014 s-1?
7HC the Quantum Mechanical Model – Download as Powerpoint Presentation (.ppt), PDF File (.pdf), Text File (.txt) or view presentation slides online. O Scribd é o …
Energy: The Bohr model and the Quantum Mechanical model of the atom both assign specific energies to an electron. In the Bohr model the energy of an …
1 7.4 The Quantum-Mechanical Model of the Atom • Bohr’s model of the H atom – Assumes the quantization without explanation – Does not take into account Heisenberg’s
from a photon of light that that “hit” the atom, we call this event an absorption event. The photon’s energy was absorbed by the electron (destroying the photon), and the electron ends up in a higher energy level. Comparison of Bohr’s Model with the Quantum (or Wave) Mechanics Model Bohr’s Model Wave Mechanics Model (Schrödinger) e-‘s are in orbits e ‘s are “in” orbitals e-in an orbit is a
12/5/2014 1 The Quantum Mechanical View of the Atom Chemistry Timeline #1 B.C. 400 B.C. Democritus and Leucippus use the term “atomos” 1500’s
Using our concept, we also mathematically derive the form of quantum mechanical wave function of free particle which is conventionally a postulate of quantum mechanics. Thus, we prove that the
View Unit 2 The Quantum Mechanical Model of the Atom.pdf from CHEM 1120 at Langara College. UNIT 2 THE QUANTUM-MECHANICAL MODEL OF THE ATOM 1 A. The Schrdinger Equation 1. Quantum mechanics (wave
The Quantum Mechanical Model of the Atom were found to exhibit certain characteristics, including diffraction (the bending of waves around corners) and interference (the adding or subtracting of energies when waves overlap).
CH40S Mr. Smith Historical Development of the Quantum-Mechanical Model Rutherford visualized the atom as a miniature solar system, with electrons circling the nucleus in
The quantum scissors device for sending qubit states of light back in time. BS1 and BS2 are 50:50 beam splitters. The rectangles are light sources and the semi-ovals are photon detectors.
The Quantum Mechanical Atom laurenhill.emsb.qc.ca
7.4 The Quantum-Mechanical Model of Atomic Orbitals the Atom
The Quantum Mechanical Atom Levels and Sublevels Images displayed in all types of media depict atoms as dense spherical nuclei surrounded by orbiting electrons.
Energy: The Bohr model and the Quantum Mechanical model of the atom both assign specific energies to an electron. In the Bohr model the energy of an …
View Unit 2 The Quantum Mechanical Model of the Atom.pdf from CHEM 1120 at Langara College. UNIT 2 THE QUANTUM-MECHANICAL MODEL OF THE ATOM 1 A. The Schrdinger Equation 1. Quantum mechanics (wave
from a photon of light that that “hit” the atom, we call this event an absorption event. The photon’s energy was absorbed by the electron (destroying the photon), and the electron ends up in a higher energy level. Comparison of Bohr’s Model with the Quantum (or Wave) Mechanics Model Bohr’s Model Wave Mechanics Model (Schrödinger) e-‘s are in orbits e ‘s are “in” orbitals e-in an orbit is a
The Quantum Mechanical Model of the Atom were found to exhibit certain characteristics, including diffraction (the bending of waves around corners) and interference (the adding or subtracting of energies when waves overlap).
1 7.4 The Quantum-Mechanical Model of the Atom • Bohr’s model of the H atom – Assumes the quantization without explanation – Does not take into account Heisenberg’s
The quantum scissors device for sending qubit states of light back in time. BS1 and BS2 are 50:50 beam splitters. The rectangles are light sources and the semi-ovals are photon detectors.
This is a test designed to help grade 12 University Chemistry students practice quantum mechanics before a big unit test. (Such as myself)
The Quantum Mechanical Model of the Hydrogen Atom (Reference: Section 7.4 (as is in the Bohr model of the hydrogen atom). Instead, the position of the electron can only be thought of in terms of the probability that an electron will be found within a certain distance of the nucleus. An atomic orbital is a region of space around a nucleus that one is likely to find an electron. The lowest
7HC the Quantum Mechanical Model – Download as Powerpoint Presentation (.ppt), PDF File (.pdf), Text File (.txt) or view presentation slides online. O Scribd é o …
Historical Development of the Quantum-Mechanical Model
A quantum mechanical model of adaptive mutation Request PDF
Bohr Model of the Atom (contd.)! • The Bohr Model led to the concept that energy is quantized: An atom cannot have a continuous range of energies (like stairs rather than a ramp).”
The Quantum Mechanical Model of the Atom howechem.net
Quantum Mechanical Model of Atom EDUREV.IN
1 7.4 The Quantum-Mechanical Model of the Atom • Bohr’s model of the H atom – Assumes the quantization without explanation – Does not take into account Heisenberg’s
ALE 4. The Quantum Mechanical Model of the Hydrogen Atom
Quantum Mechanical Model of Atom edurev.in
The Quantum Mechanical Atom laurenhill.emsb.qc.ca