Nuclear model of the atom pdf
Rutherford Atomic Model – The plum pudding model is given by J. J. Thomson failed to explain certain experimental results associated with the atomic structure of elements. Ernest Rutherford, a British scientist conducted an experiment and based on the observations of this experiment he proposed the atomic structure of elements and gave Rutherford Atomic Model.
Developing a model of the atom: the nuclear atom Students will have seen signs of electrons and positive ions (perhaps carrying a current through ionized neon or helium). On this evidence, Thomson proposed his ‘plum pudding’ model, in which the negative electrons sit in the positive nucleus like currents in a bun.
Section 44.1.1 102 Chapter 4 • The Structure of the Atom FIRE Hot Dry Wet Cold WATER AIR EARTH Objectives Compare and contrast the atomic models of Democritus,
The development of the atomic model. I love this story. It is a story of how ideas changed about the nature of the atom. These are the notes (and diagrams) I use when I teach the atomic nature of
In the space below, draw a diagram to illustrate the clay model of a typical atom you created. Label the following parts: nucleus, proton, neutron, electron cloud region.
2 LESSON PLAN (CONT.): Introducing the Atom periodictable.rosendigital.com Next Generation Science Standards Addressed MS-PS1-1. Develop models to describe the atomic composition of …
Dalton’s Model John Dalton took what was known about chemical reactions at his time and proposed the first atomic model. – Conservation of Mass
Two models of atomic structure are in use today: the Bohr model and the quantum mechanical model. The quantum mechanical model is based on mathematics. Although it is more difficult to understand than the Bohr model, it can be used to explain observations made on complex atoms.
There are different kinds of atomic orbitals that differ in the amount of energy and shapes (where the electron probably is). The atomic orbitals get filled by electrons in a certain
(ii) Extra nuclear part which contains This model was similar to the solar system. 3) Properties of the nucleus (i) Nucleus is a small, heavy, positively charged portion of the atom and located at the centre of the atom.
model atomic structure electron nucleus Models of the atom A model of the atom. But does the nucleus glow like this? No. And are electrons blue? No. The importance of these two discoveries was that they suggested that atoms were not indestructible. Atoms are tiny but they are made of still smaller particles. Positive and negative Electrons are negatively charged. However, atoms are neutral, so
(a) Around 1800, James Dalton proposed a modern atomic model, based on experimentation rather than pure reason. Describe one aspect of Dalton’s model of the atom.
Atomic and Nuclear Structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
quantum-mechanical model of the hydrogen atom The model describes an atom that has certain allowed quantities of energy due to the allowed wavelike behavior of an electronwhose
1 2.4 The Nuclear Model of the Atom • Discovery of the electron – Cathode ray tubes (cathode rays →electrons) – Mass-to-charge ratio of the electron (J.J.
model is called “the raisin bread model”; the raisins being the electrons and the atom the bread. (b) Discovery of the atomic nucleus After having made remarkable achievements in the study of radioactivity, the British physicist
However, because of its simplicity, and its correct results for selected systems see below for application , the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom.
As you may recall from Chapter 28, the need for a nuclear model of the atom was indicated by the alpha particle-scattering experiments performed around 1910. Since
Atomic number – indicates the number of protons and defines the element (atomic number 6 is always carbon, atomic number 7 is always nitrogen etc.). Mass number – equals the total number of neutrons and protons in the nucleus of an atom for the most common isotope this
Rutherford atomic model Definition & Facts Britannica.com

Chapter 5 Nuclear Shell Model Southampton
Lesson Outline Structure of the Atom Lesson Engagement 1) Coin exploration activity (5-10 min) a. Students explore various US and Canadian coins using
Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr. The Bohr model of the atom , a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models.
1 Rutherford, Radioactivity, and the Atomic Nucleus Helge Kragh* Abstract Modern atomic and nuclear physics took its start in the early part of the

Rutherford atomic model, also called nuclear atom or planetary model of the atom, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford.
The atomic mass of an atom is the sum of its protons and neutrons or Z + N. The strong nuclear force binds protons and neutrons together to form the nucleus of an atom. The strong nuclear force binds protons and neutrons together to form the nucleus of an atom.
entirely correct but it would lead to Schrodinger’s idea of the modern atomic model. 1926 – Erwin Schrödinger, an Austrian physicist, viewed electrons as continuous clouds and introduced “wave mechanics” as a mathematical model of the atom.
In this section, you will describe and explain the experimental observations that led to Rutherford’s nuclear model of the atom describe and explain the
Chapter 5 Nuclear Shell Model 5.1 Magic Numbers The binding energies predicted by the Liquid Drop Model underestimate the actual binding energies of “magic nuclei” for which either the number of neutrons N = ( A − Z) or the
Siyavula’s open Physical Sciences Grade 10 textbook, chapter 4 on The Atom
2 Atoms and the Atomic Theory W e begin this chapter with a brief survey of early chemical discov-eries, culminating in Dalton’s atomic theory. This is followed by a description of the physical evidence leading to the modern pic- ture of the nuclear atom, in which protons and neutrons are combined into a nucleus with electrons in space surrounding the nucleus. We will also introduce the
Atomic Structure Timeline •Use the following information to fill out your foldable. •You will be responsible for the information found on this PowerPoint presentation.

DO PHYSICS ONLINE FROM QUANTA TO QUARKS THE BOHR MODEL OF THE ATOM Bohr type models of the atom give a totally incorrect picture of the atom and are of only historical significance. However, the Bohr models were an important step in the development of quantum mechanics. Quantum mechanics is a mathematical theory to account for the atomic related behaviour of our physical …
Atomic Theory Timeline The atomic model has changed over time. For over two centuries, scientists have created different models of the atom. As scientists have
Rutherford’s atomic model Atomic experiment of Lord Ernest Rutherford In 1910, the New Zealand physicist Ernest Rutherford put forward the idea that the positive charges of the atom were found mostly in its center, in the nucleus , and the electrons ( e -) around it.
2.4 The Nuclear Model of the Atom g.web.umkc.edu
This allowed Rutherford to make the following discoveries: •The Rutherford model of the atom was coined the planetary model of the atom Energy Levels and the Bohr-Rutherford Model
Bohr Atomic Model : In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus.
May be used for educational purpose upon acknowledgement. © www.chemicalformula.org 3 Nuclear Model of the Atom Worksheet Name: ANSWERS ____/ 25 marks – google document pdf images not working 4.1 the atomic models of thomson and rutherford 4.2 rutherford scattering 4.3 the classic atomic model 4.4 the bohr model of the hydrogen Topic 7: Atomic and nuclear physics 7.1 The atom -. atomic structure 7.1.1 describe a model of the atom that features a …
Rutherford atomic model has been alternatively called the nuclear atom, or the planetary model of the atom. The young physicists beamed alpha particles through gold foil and detected them as flashes of light or scintillations on a screen. The gold foil was only 0.00004 centimeter thick. Most of the alpha particles went straight through the foil, but some were deflected by the foil and hit a
scientists proposed various atomic models. J.J. Thomson was the first one to propose a model for the structure of an atom. 4.2.1 THOMSON’S MODEL OF AN ATOM Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons, in a sphere of positive charge, were like currants (dry fruits) in a spherical Christmas pudding. We can also think of a watermelon, the
Atomic Structure Worksheet. Label the parts of an atom on the diagram below. 4. What type of charge does a proton have? 5. What type of charge does a neutron have? 6. What type of charge does an electron have? 7. Which two subatomic particles are located in the nucleus of an atom? 8. If an atom has 35 protons in the nucleus, how many electrons will it have orbiting the nucleus? 9. What is the
Although Thomson’s model was not an accurate model to account for the atomic structure, it proved to be the base for the development of other atomic models. Find the atomic structure pdf here. The study of the atom and its structure has paved the way for numerous inventions that have played a significant role in the development of humankind.
According to the Bohr model of the atom, the single electron of a hydrogen atom circles the nucleus a. in specific, allowed orbits. b. in one fixed orbit at all times.
Thomson 1897 Information Atomic Model Analogy In 1897, the English scientist named J.J. Thomson provided the first hint that an atom is made of even smaller particles.
2 Hu: The Cubic Atomic Model Fig. 3. A complete Helium atom with 2 neutrons This forms the Helium atom. If the Lego bricks were perfect cubes, then the Helium atom would also be a cube.
BPA BOHR MODEL L4-1 Section 4: BOHR MODEL OF THE ATOM In this section, we describe the structure and behaviour of the simplest type of atom consisting
The Case Against the Nuclear Atom by Dewey B. Larson “To all of us, steeped in the unquestioning adoration of the contemporary scientific method, this is a …
Developing a model of the atom the nuclear atom
Use the following information to fill out your foldable
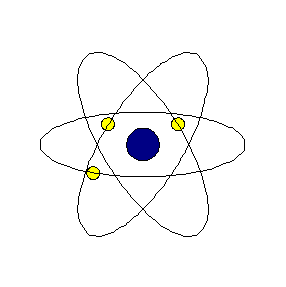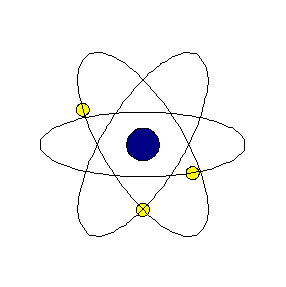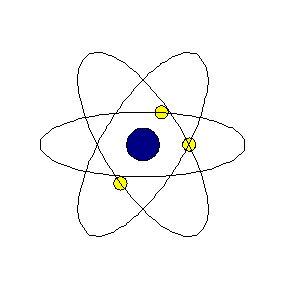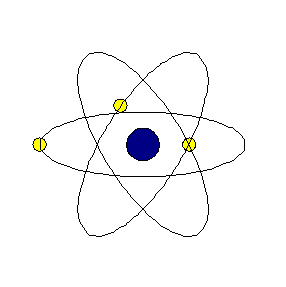
Chapter4 STRUCTURE OF THE ATOM National Council of
Atomic and Nuclear Structure Nuclear Power

Bohr Atomic Model University of Oregon
Nuclear Model of the Atom tsigaridissenior.weebly.com
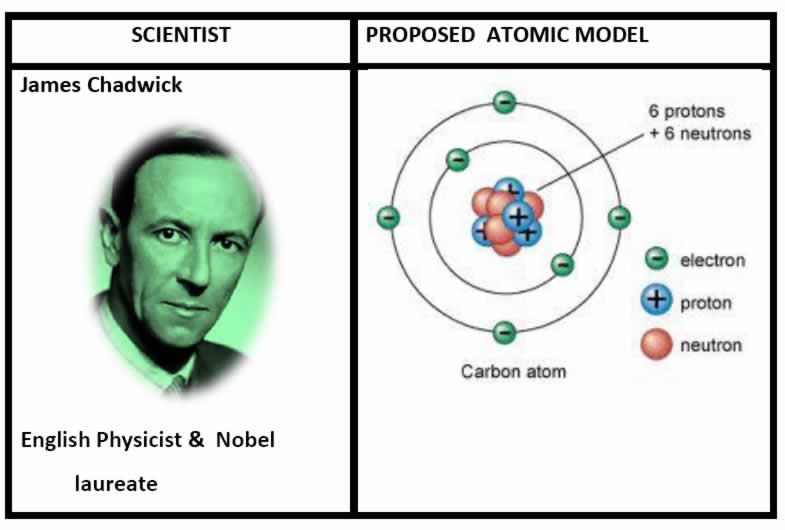
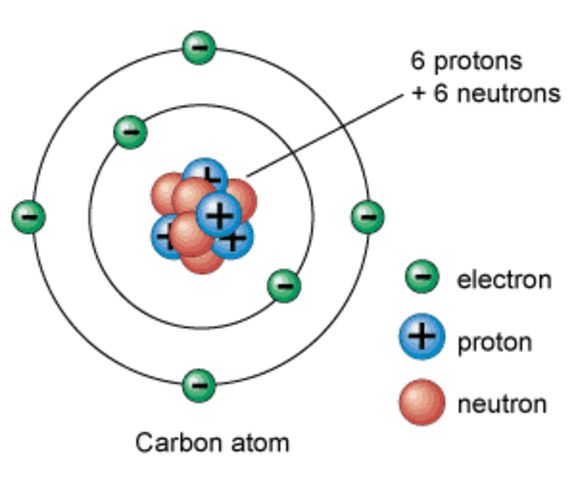
Nuclear Model of the Atom Worksheet Chemical Formula
Models of the atom STEM
– The development of the atomic model WIRED
D. B. Larson “The Case Against the Nuclear Atom”


PPT Nuclear model of atom PowerPoint Presentation – ID
Chapter4 STRUCTURE OF THE ATOM National Council of
The development of the atomic model WIRED
This allowed Rutherford to make the following discoveries: •The Rutherford model of the atom was coined the planetary model of the atom Energy Levels and the Bohr-Rutherford Model
Chapter 5 Nuclear Shell Model 5.1 Magic Numbers The binding energies predicted by the Liquid Drop Model underestimate the actual binding energies of “magic nuclei” for which either the number of neutrons N = ( A − Z) or the
1 2.4 The Nuclear Model of the Atom • Discovery of the electron – Cathode ray tubes (cathode rays →electrons) – Mass-to-charge ratio of the electron (J.J.
Lesson Outline Structure of the Atom Lesson Engagement 1) Coin exploration activity (5-10 min) a. Students explore various US and Canadian coins using
In the space below, draw a diagram to illustrate the clay model of a typical atom you created. Label the following parts: nucleus, proton, neutron, electron cloud region.
2 LESSON PLAN (CONT.): Introducing the Atom periodictable.rosendigital.com Next Generation Science Standards Addressed MS-PS1-1. Develop models to describe the atomic composition of …
QUANTUM-MECHANICAL MODEL OF THE ATOM CORE
Chapter4 STRUCTURE OF THE ATOM National Council of
In the space below, draw a diagram to illustrate the clay model of a typical atom you created. Label the following parts: nucleus, proton, neutron, electron cloud region.
Rutherford Atomic Model – The plum pudding model is given by J. J. Thomson failed to explain certain experimental results associated with the atomic structure of elements. Ernest Rutherford, a British scientist conducted an experiment and based on the observations of this experiment he proposed the atomic structure of elements and gave Rutherford Atomic Model.
quantum-mechanical model of the hydrogen atom The model describes an atom that has certain allowed quantities of energy due to the allowed wavelike behavior of an electronwhose
Dalton’s Model John Dalton took what was known about chemical reactions at his time and proposed the first atomic model. – Conservation of Mass
According to the Bohr model of the atom, the single electron of a hydrogen atom circles the nucleus a. in specific, allowed orbits. b. in one fixed orbit at all times.
Chapter 5 Nuclear Shell Model 5.1 Magic Numbers The binding energies predicted by the Liquid Drop Model underestimate the actual binding energies of “magic nuclei” for which either the number of neutrons N = ( A − Z) or the
Two models of atomic structure are in use today: the Bohr model and the quantum mechanical model. The quantum mechanical model is based on mathematics. Although it is more difficult to understand than the Bohr model, it can be used to explain observations made on complex atoms.
Developing a model of the atom: the nuclear atom Students will have seen signs of electrons and positive ions (perhaps carrying a current through ionized neon or helium). On this evidence, Thomson proposed his ‘plum pudding’ model, in which the negative electrons sit in the positive nucleus like currents in a bun.
scientists proposed various atomic models. J.J. Thomson was the first one to propose a model for the structure of an atom. 4.2.1 THOMSON’S MODEL OF AN ATOM Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons, in a sphere of positive charge, were like currants (dry fruits) in a spherical Christmas pudding. We can also think of a watermelon, the
Rutherford atomic model Definition & Facts Britannica.com
Bohr Atomic Model University of Oregon
The Case Against the Nuclear Atom by Dewey B. Larson “To all of us, steeped in the unquestioning adoration of the contemporary scientific method, this is a …
Developing a model of the atom: the nuclear atom Students will have seen signs of electrons and positive ions (perhaps carrying a current through ionized neon or helium). On this evidence, Thomson proposed his ‘plum pudding’ model, in which the negative electrons sit in the positive nucleus like currents in a bun.
Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr. The Bohr model of the atom , a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models.
Atomic and Nuclear Structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
(ii) Extra nuclear part which contains This model was similar to the solar system. 3) Properties of the nucleus (i) Nucleus is a small, heavy, positively charged portion of the atom and located at the centre of the atom.
The atomic mass of an atom is the sum of its protons and neutrons or Z N. The strong nuclear force binds protons and neutrons together to form the nucleus of an atom. The strong nuclear force binds protons and neutrons together to form the nucleus of an atom.
model atomic structure electron nucleus Models of the atom A model of the atom. But does the nucleus glow like this? No. And are electrons blue? No. The importance of these two discoveries was that they suggested that atoms were not indestructible. Atoms are tiny but they are made of still smaller particles. Positive and negative Electrons are negatively charged. However, atoms are neutral, so
scientists proposed various atomic models. J.J. Thomson was the first one to propose a model for the structure of an atom. 4.2.1 THOMSON’S MODEL OF AN ATOM Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons, in a sphere of positive charge, were like currants (dry fruits) in a spherical Christmas pudding. We can also think of a watermelon, the
(a) Around 1800, James Dalton proposed a modern atomic model, based on experimentation rather than pure reason. Describe one aspect of Dalton’s model of the atom.
This allowed Rutherford to make the following discoveries: •The Rutherford model of the atom was coined the planetary model of the atom Energy Levels and the Bohr-Rutherford Model
DO PHYSICS ONLINE FROM QUANTA TO QUARKS THE BOHR MODEL OF THE ATOM Bohr type models of the atom give a totally incorrect picture of the atom and are of only historical significance. However, the Bohr models were an important step in the development of quantum mechanics. Quantum mechanics is a mathematical theory to account for the atomic related behaviour of our physical …
The Quantum Model of the Atom D. Cassidy Books
Basic Model of the Atom and Atomic Theory ThoughtCo
The atomic mass of an atom is the sum of its protons and neutrons or Z N. The strong nuclear force binds protons and neutrons together to form the nucleus of an atom. The strong nuclear force binds protons and neutrons together to form the nucleus of an atom.
Bohr Atomic Model : In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus.
2 Hu: The Cubic Atomic Model Fig. 3. A complete Helium atom with 2 neutrons This forms the Helium atom. If the Lego bricks were perfect cubes, then the Helium atom would also be a cube.
2 Atoms and the Atomic Theory W e begin this chapter with a brief survey of early chemical discov-eries, culminating in Dalton’s atomic theory. This is followed by a description of the physical evidence leading to the modern pic- ture of the nuclear atom, in which protons and neutrons are combined into a nucleus with electrons in space surrounding the nucleus. We will also introduce the
The development of the atomic model. I love this story. It is a story of how ideas changed about the nature of the atom. These are the notes (and diagrams) I use when I teach the atomic nature of
Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr. The Bohr model of the atom , a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models.
May be used for educational purpose upon acknowledgement. © www.chemicalformula.org 3 Nuclear Model of the Atom Worksheet Name: ANSWERS ____/ 25 marks
Rutherford atomic model Definition & Facts Britannica.com
PPT Nuclear model of atom PowerPoint Presentation – ID
Siyavula’s open Physical Sciences Grade 10 textbook, chapter 4 on The Atom
scientists proposed various atomic models. J.J. Thomson was the first one to propose a model for the structure of an atom. 4.2.1 THOMSON’S MODEL OF AN ATOM Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons, in a sphere of positive charge, were like currants (dry fruits) in a spherical Christmas pudding. We can also think of a watermelon, the
Section 44.1.1 102 Chapter 4 • The Structure of the Atom FIRE Hot Dry Wet Cold WATER AIR EARTH Objectives Compare and contrast the atomic models of Democritus,
Atomic Structure Worksheet. Label the parts of an atom on the diagram below. 4. What type of charge does a proton have? 5. What type of charge does a neutron have? 6. What type of charge does an electron have? 7. Which two subatomic particles are located in the nucleus of an atom? 8. If an atom has 35 protons in the nucleus, how many electrons will it have orbiting the nucleus? 9. What is the
model atomic structure electron nucleus Models of the atom A model of the atom. But does the nucleus glow like this? No. And are electrons blue? No. The importance of these two discoveries was that they suggested that atoms were not indestructible. Atoms are tiny but they are made of still smaller particles. Positive and negative Electrons are negatively charged. However, atoms are neutral, so
BPA BOHR MODEL L4-1 Section 4: BOHR MODEL OF THE ATOM In this section, we describe the structure and behaviour of the simplest type of atom consisting
There are different kinds of atomic orbitals that differ in the amount of energy and shapes (where the electron probably is). The atomic orbitals get filled by electrons in a certain
Atomic and Nuclear Structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
May be used for educational purpose upon acknowledgement. © www.chemicalformula.org 3 Nuclear Model of the Atom Worksheet Name: ANSWERS ____/ 25 marks
Thomson 1897 Information Atomic Model Analogy In 1897, the English scientist named J.J. Thomson provided the first hint that an atom is made of even smaller particles.
Lesson Outline Structure of the Atom Lesson Engagement 1) Coin exploration activity (5-10 min) a. Students explore various US and Canadian coins using
4.1 the atomic models of thomson and rutherford 4.2 rutherford scattering 4.3 the classic atomic model 4.4 the bohr model of the hydrogen Topic 7: Atomic and nuclear physics 7.1 The atom -. atomic structure 7.1.1 describe a model of the atom that features a …
2 LESSON PLAN (CONT.): Introducing the Atom periodictable.rosendigital.com Next Generation Science Standards Addressed MS-PS1-1. Develop models to describe the atomic composition of …
Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr. The Bohr model of the atom , a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models.
DO PHYSICS ONLINE FROM QUANTA TO QUARKS THE BOHR MODEL OF THE ATOM Bohr type models of the atom give a totally incorrect picture of the atom and are of only historical significance. However, the Bohr models were an important step in the development of quantum mechanics. Quantum mechanics is a mathematical theory to account for the atomic related behaviour of our physical …
Atomic and Nuclear Structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
Chapter 5 Nuclear Shell Model Southampton
2.4 The Nuclear Model of the Atom g.web.umkc.edu
Chapter 4 The Structure of the Atom Mrs.Taylor’s Classes