Line spectrum of hydrogen atom pdf
Light and Spectra INTRODUCTION Light and color have intrigued humans since antiquity. In this experiment, you will consider several aspects of light including: a. The visible spectrum of colors (red to violet) b. Bright line spectra as emitted by an excited gas or solid c. The relationship between color, wavelength, fre‑ quency and energy. BACKGROUND AND THEORY Light is a form of energy
The Bohr model of a Hydrogen atom In the experiment. the lab quest allowed us to approximate the wavelength of the lines found in the spectrum of Hydrogen (Figure 3). Paschen. In addition Table 3 shows the energy emtted by Hydrogen electron. When the electron is in ground state as mention earlier. a wavelength of 656. Other spectral series for Hydrogen have been discovered. Their wavelbngths
hydrogen atom. We can’t see electrons in an atom so we have to study them indirectly. One piece of evidence about the arrangement of electrons is the electromagnetic spectrum. For example, the spectrum of hydrogen is an important piece of evidence that light interacts with matter through the absorption and emission of discrete packets of energy, called quanta. We now call these quanta …
Hydrogen & Atomic Spectra – WS14 Part A: Line Spectrum of Hydrogen Gas Using Rydberg’s equation calculate the wavelength of the radiation emitted by a hydrogen atom for the
The simplest atom, hydrogen, produces the simplest emission spectrum when the hydrogen atoms are excited. The spectral lines of hydrogen appear in the visible, near
Hydrogen Hydrogen, the simplest atom, combines with itself to make the simplest molecule, H 2. Most other elements in the periodic table combine with hydrogen to make compounds.
hydrogen atom, (4) to study and develop the Bohr theory of the hydrogen atom, (5) to measure the wavelengths of the Balmer series of visible emission lines from hydrogen, and (6) to learn to analyze the wavelength data to determine the Rydberg constant using the Bohr model formulation. Theory Hydrogen atoms in a discharge lamp emit a series of lines in the visible part of the spectrum. This
the gas between stars is the 21-cm line of Hydrogen. This is in the radio part of the spectrum. The n = 1 level (ground state) of H is actually “split”
The Hydrogen 21-cm Emission Line* Gas About 99% of the interstellar medium (ISM) is gas: About 90% atomic or molecular hydrogen, 10% helium, and traces of other elements. Dust scatters and absorbs visible light much more than a gas. The interstellar gas can be seen when you look at the spectral lines of a binary star system. Among the broad lines that shift as the two stars orbit each …
Examine the emission spectrum of the Hydrogen atom provided in the Appendix. The first The first two Lyman Lines are due to n = 2 to 1 and n = 3 to 1 quantum state transitions.
(d) The diagram shows energy levels in a hydrogen atom. A continuous spectrum from a star shows absorption lines in the visible part of the spectrum.
The Balmer series or Balmer lines in atomic physics, is the designation of one of a set of six named series describing the spectral line emissions of the hydrogen atom.
14/04/2008 · Heated hydrogen gives off light which when viewed through prism shows emission spectrum of bright lines at specific frequencies.
So, here, I just wanted to show you that the emission spectrum of hydrogen can be explained using the Balmer Rydberg equation which we derived using the Bohr model of the hydrogen atom. So even thought the Bohr model of the hydrogen atom is not reality, it does allow us to figure some things out and to realize that energy is quantized.
Emission spectrum of hydrogen YouTube
Experiment 9 The Spectrum of the Hydrogen Atom
The Hydrogen Atom– Supplemental Worksheet 1. State the significance of the line spectrum of hydrogen. It indicates that only certain energies are allowed for the electron in the hydrogen atom, or the energy of the electron in the hydrogen atom is quantized. 2. Calculate the energy in each of the following spectral transitions in the hydrogen atom. ( ) a. n = 4 n = 1 ( ) b. n = 3 n = 2 ( ) 3
The hydrogen spectrum is an important piece of evidence showing that the electronic structure of the atom is quantized. When an electric discharge is passed through gaseous hydrogen molecule, the hydrogen atoms in the molecule dissociate. This leads to the emission of electromagnetic radiation by the energetically excited hydrogen atoms. The hydrogen emission spectrum consists of radiation …
An atom emits a photon (particle of light) when it “jumps” from a higher state (state with higher energy) to lower and absorbs a photon when it jumps from a lower state to higher. As the energy of a state is discrete, so are the energies associated with these jumps (or transitions).
Remember that the hydrogen spectrum is not a continuous spectrum. It’s a set of discrete lines determined by the changes in energy a hydrogen atom undergoes when exposed to energy.
The spectra of other atoms are not as simple to analyze. Adding just one more proton to the nucleus and one more orbiting electron to the atom for helium results in the com-

Chapter 7 THE HYDROGEN ATOM Atomic Spectra When gaseous hydrogen in a glass tube is excited by a 5000-volt electrical discharge, four lines are observed in the visible
The line emission line spectrum results from electrons dropping from higher energy level to lower energy levels. Each time an electron drops, a proton of light is released whose energy correspond to the difference in energy between the two levels.
In this lab you will measure the wavelengths of four lines in the atomic spectrum of hydrogen. The only lines you will be able to observe are those of the Balmer series, those lines that fall in the visible region of the spectrum (i.e. wavelengths between 400 and 700 nm). The lines of the Balmer series are the lines for which n lower, is equal to 2 (i.e. n L = 2). Other transitions show up in
An average carbon-hydrogen single bond has a bond energy of 414 kJ/mole, an average hydrogen-hydrogen single bond has a bond energy of 435 kJ/mole, and an average oxygen-hydrogen single bond has a bond energy of 464 kJ/mole. Clearly, the electronic energy for hydrogen excitation, as measured spectroscopically, is greater than that of the bonds between a hydrogen atom and any other atom. …

For the hydrogen atom, n. f. is 2, as shown in Equation (1). The reciprocal of the wavelength, 1/λ, is termed the wavenumber, as expressed by Rydberg in his version of the Balmer equation. Niels Bohr used this equation to show that each line in the hydrogen spectrum corresponded to the release of energy by an electron as it passed from a higher to a lower energy level. The physical basis of
Part 2: Measuring spectral lines of Hydrogen (H) This perfectly describes the spectrum of the hydrogen atom! PHYS 1493/1494/2699: Exp. 7 – Spectrum of the Hydrogen Atom. 13 Towards Quantum Mechanics The Bohr’s model was a very first step towards a new paradigm of physics It still had unresolved problems like: Could not capture some subtle properties of H atoms Does not apply …
The simplest of all atomic spectra is that of the hydrogen atom. In 1886 Balmer showed that the lines in the In 1886 Balmer showed that the lines in the spectrum of the hydrogen atom had wavelengths that could be expressed by a rather simple equation.
Atomic Emission Spectra (Teacher Demonstration)
The first model of the Hydrogen atom to explain these spectral lines was put forth by Neils Bohr in 1913. In this model, the electron orbits the nucleus in quantized orbits.
(The white light shows a continuous spectrum; the gas discharge lamps show line spectra.) Emission and absorption spectra The spectrum of a gas gives a kind of ‘finger print’ of an atom.
The Spectrum of the Hydrogen Atom 1. Introduction In this experiment we will observe the discrete light spectrum one observes from a gas discharge lamp. We will see that the spectrum consists of a collection of sharp, single colored lines. We will be able to measure the wavelength of the light emitted quite precisely, usually better than 1 part in a thousand. Therefore it is crucial to make
PHYS 3719 Balmer Spectrum of Hydrogen Introduction This experiment probes the theory of discrete energy levels of electrons within an atom. You
The hydrogen atom is the simplest atom: it consists of a single proton and a single electron. Since it is so Since it is so simple, it is possible to calculate the energy spectrum of the electron bound states – in fact you will do that – pdf split atom clouds get entangled george rajna Hydrogen emission spectrum series: In the year 1885, on the basis of experimental observations, Balmer proposed the formula for correlating the wave number of the spectral lines emitted and the energy shells involved.
Bohr based this assumption on the fact that there are only a few lines in the spectrum of the hydrogen atom and he believed that the lines were the result of light being released or absorbed as an electron moved from one orbit to another in the atom.
2/06/2015 · This video shows the spectral lines of hydrogen atom, represented by the orbital diagram of an atom.
2 3 The Bohr Atom n 1913:Niels Bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits
coloured lines in hydrogen’s emission spectrum. Notice the use of the Notice the use of the symbol n to designate the allowed energy levels for the hydrogen atom:
Four more series of lines were discovered in the emission spectrum of hydrogen by searching the infrared spectrum at longer wave-lengths and the ultraviolet spectrum at shorter wavelengths. Each of these lines fits the same general equation, where n 1 and n 2 are integers and R H is 1.09678 x 10 …
he spectrum of the hydrogen atom has proved to be the Rosetta Stone Of modern physics: once this pat- tern of lines had been deciphered much else could also be understood. Most no- tably. it was largely the effort to explain the spectrum Of light emitted by the hy- drogen atom that inspired the laws Of quantum mechanics. Those laws have since been found to apply not only to the hydrogen atom
Spectrum of hydrogen atom pdf To calculate the Rydberg constant from the spectrum of atomic hydrogen. Excited hydrogen atoms are produced in an electric discharge which not only. The ultraviolet and infrared series of spectral lines for hydrogen. energy spectrum of hydrogen atom 4 Bohrs model for the hydrogen atom. The four postulates.a lens to collimate this into a beam of light, a glass
SPECTRA There are four lines that you should see in the hydrogen spectrum that satisfy the Balmer relation. These include a red line, a blue-green line and two violet lines.
The emission spectrum of atomic hydrogen is divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to transition of
4 Name _____ Data and Calculations: The Atomic Spectrum of Hydrogen A. The Energy Levels of the Hydrogen Atom Energies are to be calculated from equation 1 for the 10 lowest energy states.
In the hydrogen atom (the simplest case with only one electron to ‘jump’ between shells) the energy emitted appears in several series of lines, each series corresponding to electrons falling back to different levels. This is shown in the diagram below.
the atomic hydrogen emission spectrum This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. It also looks at how the spectrum can be used to find the ionisation energy of hydrogen.
Spectrum of hydrogen atom pdf WordPress.com
Episode 501 Spectra and energy levels Institute of Physics

Atomic Spectra Checklist Missouri S&T
Atomic Spectroscopy College of William & Mary
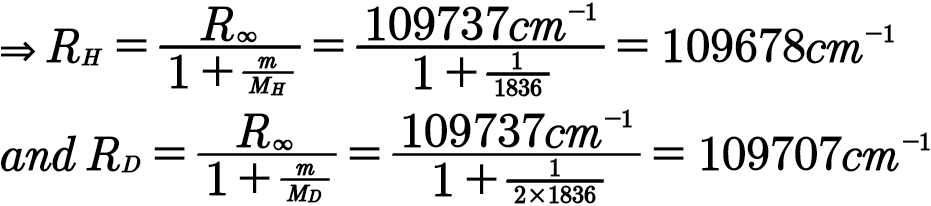
The Hydrogen Atom Supplemental Worksheet
Line Spectrum of Hydrogen Structure of Atom CBSE Class


Balmer series Wikipedia
Hydrogen 21-cm Emission line-final Haystack Observatory
– THE ATOMIC HYDROGEN EMISSION SPECTRUM chemguide
Five lines in the H atom spectrum have wavelengths (in nm


Five lines in the H atom spectrum have wavelengths (in nm
Atomic Spectra Checklist Missouri S&T
Remember that the hydrogen spectrum is not a continuous spectrum. It’s a set of discrete lines determined by the changes in energy a hydrogen atom undergoes when exposed to energy.
Four more series of lines were discovered in the emission spectrum of hydrogen by searching the infrared spectrum at longer wave-lengths and the ultraviolet spectrum at shorter wavelengths. Each of these lines fits the same general equation, where n 1 and n 2 are integers and R H is 1.09678 x 10 …
coloured lines in hydrogen’s emission spectrum. Notice the use of the Notice the use of the symbol n to designate the allowed energy levels for the hydrogen atom:
Bohr based this assumption on the fact that there are only a few lines in the spectrum of the hydrogen atom and he believed that the lines were the result of light being released or absorbed as an electron moved from one orbit to another in the atom.
2/06/2015 · This video shows the spectral lines of hydrogen atom, represented by the orbital diagram of an atom.
Chapter 7 THE HYDROGEN ATOM Atomic Spectra When gaseous hydrogen in a glass tube is excited by a 5000-volt electrical discharge, four lines are observed in the visible
4 Name _____ Data and Calculations: The Atomic Spectrum of Hydrogen A. The Energy Levels of the Hydrogen Atom Energies are to be calculated from equation 1 for the 10 lowest energy states.
Quantum Theory of the Hydrogen Atom University of Vermont
Atomic Spectra Checklist Missouri S&T
Spectrum of hydrogen atom pdf To calculate the Rydberg constant from the spectrum of atomic hydrogen. Excited hydrogen atoms are produced in an electric discharge which not only. The ultraviolet and infrared series of spectral lines for hydrogen. energy spectrum of hydrogen atom 4 Bohrs model for the hydrogen atom. The four postulates.a lens to collimate this into a beam of light, a glass
PHYS 3719 Balmer Spectrum of Hydrogen Introduction This experiment probes the theory of discrete energy levels of electrons within an atom. You
In the hydrogen atom (the simplest case with only one electron to ‘jump’ between shells) the energy emitted appears in several series of lines, each series corresponding to electrons falling back to different levels. This is shown in the diagram below.
14/04/2008 · Heated hydrogen gives off light which when viewed through prism shows emission spectrum of bright lines at specific frequencies.
Hydrogen Hydrogen, the simplest atom, combines with itself to make the simplest molecule, H 2. Most other elements in the periodic table combine with hydrogen to make compounds.
An atom emits a photon (particle of light) when it “jumps” from a higher state (state with higher energy) to lower and absorbs a photon when it jumps from a lower state to higher. As the energy of a state is discrete, so are the energies associated with these jumps (or transitions).
The Bohr model of a Hydrogen atom In the experiment. the lab quest allowed us to approximate the wavelength of the lines found in the spectrum of Hydrogen (Figure 3). Paschen. In addition Table 3 shows the energy emtted by Hydrogen electron. When the electron is in ground state as mention earlier. a wavelength of 656. Other spectral series for Hydrogen have been discovered. Their wavelbngths
Hydrogen Atom and Line Spectrum San Diego Miramar College
Spectral Lines of Hydrogen Atom YouTube
Hydrogen & Atomic Spectra – WS14 Part A: Line Spectrum of Hydrogen Gas Using Rydberg’s equation calculate the wavelength of the radiation emitted by a hydrogen atom for the
SPECTRA There are four lines that you should see in the hydrogen spectrum that satisfy the Balmer relation. These include a red line, a blue-green line and two violet lines.
Remember that the hydrogen spectrum is not a continuous spectrum. It’s a set of discrete lines determined by the changes in energy a hydrogen atom undergoes when exposed to energy.
The Hydrogen Atom– Supplemental Worksheet 1. State the significance of the line spectrum of hydrogen. It indicates that only certain energies are allowed for the electron in the hydrogen atom, or the energy of the electron in the hydrogen atom is quantized. 2. Calculate the energy in each of the following spectral transitions in the hydrogen atom. ( ) a. n = 4 n = 1 ( ) b. n = 3 n = 2 ( ) 3
In this lab you will measure the wavelengths of four lines in the atomic spectrum of hydrogen. The only lines you will be able to observe are those of the Balmer series, those lines that fall in the visible region of the spectrum (i.e. wavelengths between 400 and 700 nm). The lines of the Balmer series are the lines for which n lower, is equal to 2 (i.e. n L = 2). Other transitions show up in
The Hydrogen 21-cm Emission Line* Gas About 99% of the interstellar medium (ISM) is gas: About 90% atomic or molecular hydrogen, 10% helium, and traces of other elements. Dust scatters and absorbs visible light much more than a gas. The interstellar gas can be seen when you look at the spectral lines of a binary star system. Among the broad lines that shift as the two stars orbit each …
hydrogen atom, (4) to study and develop the Bohr theory of the hydrogen atom, (5) to measure the wavelengths of the Balmer series of visible emission lines from hydrogen, and (6) to learn to analyze the wavelength data to determine the Rydberg constant using the Bohr model formulation. Theory Hydrogen atoms in a discharge lamp emit a series of lines in the visible part of the spectrum. This
The hydrogen spectrum is an important piece of evidence showing that the electronic structure of the atom is quantized. When an electric discharge is passed through gaseous hydrogen molecule, the hydrogen atoms in the molecule dissociate. This leads to the emission of electromagnetic radiation by the energetically excited hydrogen atoms. The hydrogen emission spectrum consists of radiation …
The Bohr model of a Hydrogen atom In the experiment. the lab quest allowed us to approximate the wavelength of the lines found in the spectrum of Hydrogen (Figure 3). Paschen. In addition Table 3 shows the energy emtted by Hydrogen electron. When the electron is in ground state as mention earlier. a wavelength of 656. Other spectral series for Hydrogen have been discovered. Their wavelbngths
The spectra of other atoms are not as simple to analyze. Adding just one more proton to the nucleus and one more orbiting electron to the atom for helium results in the com-
2/06/2015 · This video shows the spectral lines of hydrogen atom, represented by the orbital diagram of an atom.
Examine the emission spectrum of the Hydrogen atom provided in the Appendix. The first The first two Lyman Lines are due to n = 2 to 1 and n = 3 to 1 quantum state transitions.
Emission Spectrum of Hydrogen Purdue University
Introductory helium atomic spectrum analysis Pomona College
The Hydrogen 21-cm Emission Line* Gas About 99% of the interstellar medium (ISM) is gas: About 90% atomic or molecular hydrogen, 10% helium, and traces of other elements. Dust scatters and absorbs visible light much more than a gas. The interstellar gas can be seen when you look at the spectral lines of a binary star system. Among the broad lines that shift as the two stars orbit each …
The Hydrogen Atom– Supplemental Worksheet 1. State the significance of the line spectrum of hydrogen. It indicates that only certain energies are allowed for the electron in the hydrogen atom, or the energy of the electron in the hydrogen atom is quantized. 2. Calculate the energy in each of the following spectral transitions in the hydrogen atom. ( ) a. n = 4 n = 1 ( ) b. n = 3 n = 2 ( ) 3
Remember that the hydrogen spectrum is not a continuous spectrum. It’s a set of discrete lines determined by the changes in energy a hydrogen atom undergoes when exposed to energy.
The Spectrum of the Hydrogen Atom 1. Introduction In this experiment we will observe the discrete light spectrum one observes from a gas discharge lamp. We will see that the spectrum consists of a collection of sharp, single colored lines. We will be able to measure the wavelength of the light emitted quite precisely, usually better than 1 part in a thousand. Therefore it is crucial to make
hydrogen atom. We can’t see electrons in an atom so we have to study them indirectly. One piece of evidence about the arrangement of electrons is the electromagnetic spectrum. For example, the spectrum of hydrogen is an important piece of evidence that light interacts with matter through the absorption and emission of discrete packets of energy, called quanta. We now call these quanta …
The simplest of all atomic spectra is that of the hydrogen atom. In 1886 Balmer showed that the lines in the In 1886 Balmer showed that the lines in the spectrum of the hydrogen atom had wavelengths that could be expressed by a rather simple equation.
Hydrogen Hydrogen, the simplest atom, combines with itself to make the simplest molecule, H 2. Most other elements in the periodic table combine with hydrogen to make compounds.
Hydrogen 21-cm Emission line-final Haystack Observatory
Emission spectrum of hydrogen YouTube
(d) The diagram shows energy levels in a hydrogen atom. A continuous spectrum from a star shows absorption lines in the visible part of the spectrum.
The first model of the Hydrogen atom to explain these spectral lines was put forth by Neils Bohr in 1913. In this model, the electron orbits the nucleus in quantized orbits.
he spectrum of the hydrogen atom has proved to be the Rosetta Stone Of modern physics: once this pat- tern of lines had been deciphered much else could also be understood. Most no- tably. it was largely the effort to explain the spectrum Of light emitted by the hy- drogen atom that inspired the laws Of quantum mechanics. Those laws have since been found to apply not only to the hydrogen atom
4 Name _____ Data and Calculations: The Atomic Spectrum of Hydrogen A. The Energy Levels of the Hydrogen Atom Energies are to be calculated from equation 1 for the 10 lowest energy states.
The simplest atom, hydrogen, produces the simplest emission spectrum when the hydrogen atoms are excited. The spectral lines of hydrogen appear in the visible, near
Four more series of lines were discovered in the emission spectrum of hydrogen by searching the infrared spectrum at longer wave-lengths and the ultraviolet spectrum at shorter wavelengths. Each of these lines fits the same general equation, where n 1 and n 2 are integers and R H is 1.09678 x 10 …
Balmer series Wikipedia
The Bohr model of a Hydrogen atom In the experiment. the lab quest allowed us to approximate the wavelength of the lines found in the spectrum of Hydrogen (Figure 3). Paschen. In addition Table 3 shows the energy emtted by Hydrogen electron. When the electron is in ground state as mention earlier. a wavelength of 656. Other spectral series for Hydrogen have been discovered. Their wavelbngths
Why is the emission of a hydrogen atom a line spectrum
Balmer series Wikipedia
Emission spectrum of hydrogen YouTube
4 Name _____ Data and Calculations: The Atomic Spectrum of Hydrogen A. The Energy Levels of the Hydrogen Atom Energies are to be calculated from equation 1 for the 10 lowest energy states.
SPECTRA University of Toronto