Historical development of atom pdf
The History of the Atom Timeline: 400 BC Scientist: Democritus (Greek Philosopher) Democritus was a Greek philosopher who was Each atom (of an element) is different in structure from other atoms (of other elements) An atom can be divided in smaller subatomic particles: Protons, Electrons and Neutrons The nucleus is the centre of an atom. It contains protons and neutrons. Electrons orbit
History leading to the creation of the atomic bomb Atomic science began many centuries ago with experimenting and probing into the nature and structure of matter. This …
History of the Atom – Worksheet/Review sheet Name: Answer Key Period:-1. State 2 similarities between Dalton’s and the Modern Day model of the atom.
Dalton reintroduced the concept of the atom and created _____ which was the basic concept of the molecule.
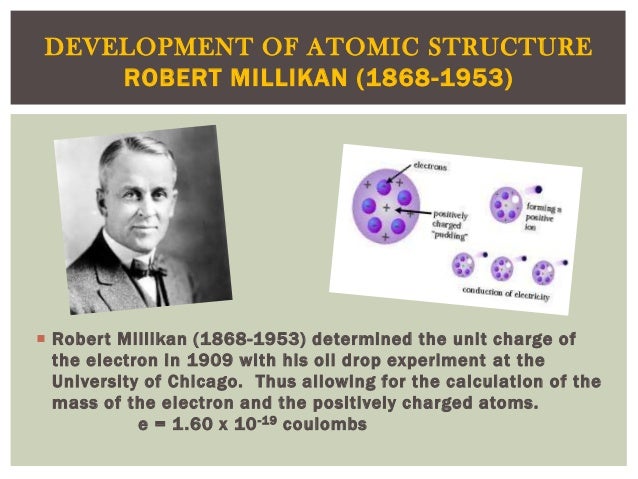
Calculated that the atom consisted mostly of empty space through which electrons move and also concluded that there was a tiny, dense region “the nucleus” centrally located within the atom that contained all of an atom’s positive charge and virtually all of its mass.
Intel Chips Throughout Intel’s history, new and improved technologies have transformed the human experience. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
Historical Development Since Roman times matter had been viewed by some as discrete particles somehow linked together. Early in the eighteenth century the behavior of gases was viewed as a function of kinetic theory .
– Historical Development Valproic acid has an analogue found in nature in the form of the valerian plant. This chemical was artificially synthesized in 1882 using the chemical in valerian as a reference. The chemical is a liquid at room temperature and was and was for a long time employed as a chemically inert solvent. Application for the drug was discovered as an anticonvulsant and
Purpose. To explore the early history of the periodic table and how it contributed to the understanding of atoms. Context. This lesson is the third of a five-part series that will broaden and enhance students’ understanding of the atom and the history of its discovery and development …
Chemistry: Development of the Atomic Theory. STUDY. PLAY. More than 2000 years ago, Greek philosophers proposed the existence of very small, indivisible particles, each of which is called an . atom. The theory that such particles existed was supported, much later by , who proposed, in his law of , that matter can’t cannot be created or destroyed. Lavoisier Conservation of matter. Then Proposed
Key Historical Developments and Experiments that reveal Atoms and Atomic Structure The idea of the atom is developed: Around 400 B.C. Greek philosophers Leucippus and Democritus asserted that all material things are
3 John Dalton (1766 – 1844) father of the atomic concept. The main points of Dalton’s atomic theory were: • Elements are made of extremely small particles called atoms.
For example, water is a compound made up of 2 atoms of hydrogen and 1 atom of oxygen (a ratio of 2:1). Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water. Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water.
a Historical Perspective Early Greek Theories 400 B.C. – Democritus thought matter could not be divided indefinitely. John Dalton 1800 -Dalton proposed a modern atomic model based on experimentation not on pure reason.
Dalton and many others continued to believe that water particles contained one hydrogen atom and one oxygen atom, rather than two hydrogen atoms and one oxygen atom. The historical development of the concept of the atom is summarized in Figure 1.15 “A Summary of the Historical Development of the Concept of the Atom” .
Early History of the Atom Matter is composed of indivisible building blocks. This idea was recorded as early as the fifth century BCE by Leucippus and Democritus.
Historical Development of Atom & The Chemical Prezi
(PDF) Historical development of knowledge about the atom
Historical Development of Atom & The Chemical Element Why Prezi. The science Conversational presenting
history, scientists have accepted five major different atomic models. Our perception of the Our perception of the atom has changed from the early Greek model because of …
The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis of …
PDF On Jan 1, 2009, Zejnilagić-Hajrić Meliha and others published Historical development of knowledge about the atom
Historical Development of Atomic Theory: Aristotle to Rutherford1. Democritus and Aristotle in 400 B.C.2. Aristotle: matter can be divided into smaller andsmaller particles.3. Democritus: the atom is the smallest unit.4. Aristotle’s theory was most popular.
Summary of the Historical Development of Modern Theory of Atomic Structure The table below summarises the contribution of a number of Scientists to the development of a model of the atom including the evidence they used to support the model.
The Development of Dalton’s Atomic Theory as a Case Study in the History of Science: Reflections for Educators in Chemistry He´lio Elael Bonini Viana Æ Paulo Alves Porto Published online: 4 February 2009 Springer Science+Business Media B.V. 2009 Abstract The inclusion of the history of science in science curricula—and specially, in the curricula of science teachers—is a trend that has
Abstract. The genesis and historical background of the hydro-gen bomb are described with particular emphasis placed on the development of physical ideas which led to the discovery of the
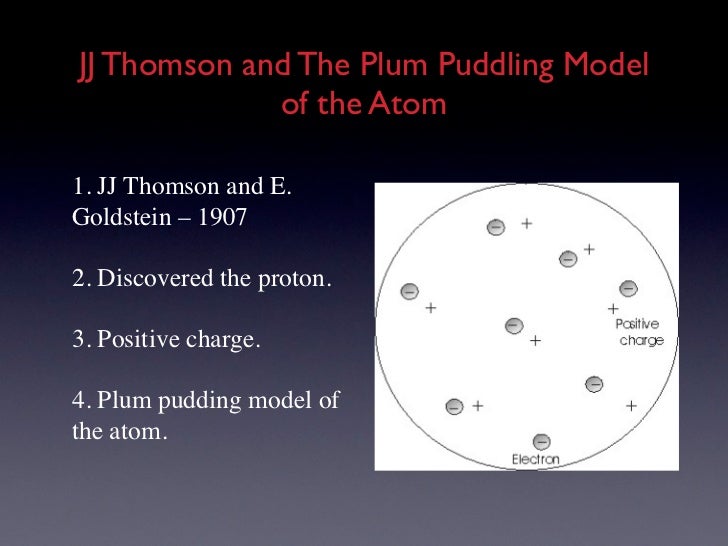
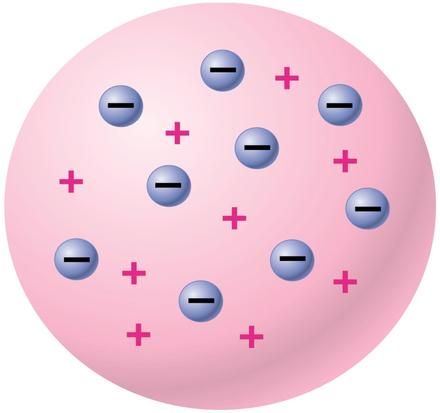
He also proposed that all of the positive charge and all of the mass of the atom occupied a small space in the center of an atom and that most of the atom is empty space occupied by electrons (Historical …
Draw a diagram of an atom labeling the proton, neutron, and electron. (1 pts) (1 pts) With your group, design a poster or power point presentation of your findings.
historical studies has taught us about historical economic growth and development. 7.1.1 TheOriginsoftheLiterature The origins of the historical development literature can …
2.1 Historical Development of Atomic Theory. 2 2.1.1 The Periodic Table of the Elements 2.1.2 Discovery of Subatomic Particles & the Bohr Atom Each element emits light of specific energies when excited by electric discharge or heat. For the H-atom (Balmer, 1885): 6 5 4 3 2 n = 3 2.1.2 Discovery of Subatomic Particles & the Bohr Atom Johann Jacob Balmer (Physicist, 1825 – 1899) The Hydrogen
HISTORICAL OUTLINE of the Atomic Theory and the Structure of the Atom . Development of the Atomic Theory . Democritus (460-370 BC) First proposed the existence of an ultimate particle. Used the word “atomos” to describe this particle. Democritus . Aristotle (384-322 BC) was a proponent of the continuum. He believed in the four elements of air, earth, water and fire. Aristotle felt that

Historical Development of the Atom Model
– create pdf from images windows
2.1 Historical Development of Atomic Theory
History of Atomic Structure Boundless Chemistry

History Of The Atom ProProfs Quiz
History Of Chemistry ProProfs Quiz

History of Atomic Theory and the Structure of the Atom
Intel Chips timeline
Molecules Chemistry Encyclopedia – structure water
marquee tag in html for multiple images with example 2 –


(PDF) Historical development of knowledge about the atom
Historical Development of Atomic Theory SlideShare
HISTORICAL OUTLINE of the Atomic Theory and the Structure of the Atom . Development of the Atomic Theory . Democritus (460-370 BC) First proposed the existence of an ultimate particle. Used the word “atomos” to describe this particle. Democritus . Aristotle (384-322 BC) was a proponent of the continuum. He believed in the four elements of air, earth, water and fire. Aristotle felt that
Early History of the Atom Matter is composed of indivisible building blocks. This idea was recorded as early as the fifth century BCE by Leucippus and Democritus.
Historical Development of Atom & The Chemical Element Why Prezi. The science Conversational presenting
history, scientists have accepted five major different atomic models. Our perception of the Our perception of the atom has changed from the early Greek model because of …
The History of the Atom Timeline: 400 BC Scientist: Democritus (Greek Philosopher) Democritus was a Greek philosopher who was Each atom (of an element) is different in structure from other atoms (of other elements) An atom can be divided in smaller subatomic particles: Protons, Electrons and Neutrons The nucleus is the centre of an atom. It contains protons and neutrons. Electrons orbit
Intel Chips Throughout Intel’s history, new and improved technologies have transformed the human experience. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
For example, water is a compound made up of 2 atoms of hydrogen and 1 atom of oxygen (a ratio of 2:1). Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water. Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water.
Purpose. To explore the early history of the periodic table and how it contributed to the understanding of atoms. Context. This lesson is the third of a five-part series that will broaden and enhance students’ understanding of the atom and the history of its discovery and development …
History Of The Atom ProProfs Quiz
Atomic History Project Patrick County Public Schools
Historical Development of Atomic Theory: Aristotle to Rutherford1. Democritus and Aristotle in 400 B.C.2. Aristotle: matter can be divided into smaller andsmaller particles.3. Democritus: the atom is the smallest unit.4. Aristotle’s theory was most popular.
For example, water is a compound made up of 2 atoms of hydrogen and 1 atom of oxygen (a ratio of 2:1). Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water. Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water.
Chemistry: Development of the Atomic Theory. STUDY. PLAY. More than 2000 years ago, Greek philosophers proposed the existence of very small, indivisible particles, each of which is called an . atom. The theory that such particles existed was supported, much later by , who proposed, in his law of , that matter can’t cannot be created or destroyed. Lavoisier Conservation of matter. Then Proposed
3 John Dalton (1766 – 1844) father of the atomic concept. The main points of Dalton’s atomic theory were: • Elements are made of extremely small particles called atoms.
– Historical Development Valproic acid has an analogue found in nature in the form of the valerian plant. This chemical was artificially synthesized in 1882 using the chemical in valerian as a reference. The chemical is a liquid at room temperature and was and was for a long time employed as a chemically inert solvent. Application for the drug was discovered as an anticonvulsant and
He also proposed that all of the positive charge and all of the mass of the atom occupied a small space in the center of an atom and that most of the atom is empty space occupied by electrons (Historical …
historical studies has taught us about historical economic growth and development. 7.1.1 TheOriginsoftheLiterature The origins of the historical development literature can …
Abstract. The genesis and historical background of the hydro-gen bomb are described with particular emphasis placed on the development of physical ideas which led to the discovery of the
The Development of Dalton’s Atomic Theory as a Case Study
Historical Development of Atomic Theory SlideShare
Purpose. To explore the early history of the periodic table and how it contributed to the understanding of atoms. Context. This lesson is the third of a five-part series that will broaden and enhance students’ understanding of the atom and the history of its discovery and development …
The Development of Dalton’s Atomic Theory as a Case Study in the History of Science: Reflections for Educators in Chemistry He´lio Elael Bonini Viana Æ Paulo Alves Porto Published online: 4 February 2009 Springer Science Business Media B.V. 2009 Abstract The inclusion of the history of science in science curricula—and specially, in the curricula of science teachers—is a trend that has
Key Historical Developments and Experiments that reveal Atoms and Atomic Structure The idea of the atom is developed: Around 400 B.C. Greek philosophers Leucippus and Democritus asserted that all material things are
Summary of the Historical Development of Modern Theory of Atomic Structure The table below summarises the contribution of a number of Scientists to the development of a model of the atom including the evidence they used to support the model.
History of the Atom – Worksheet/Review sheet Name: Answer Key Period:-1. State 2 similarities between Dalton’s and the Modern Day model of the atom.
Dalton and many others continued to believe that water particles contained one hydrogen atom and one oxygen atom, rather than two hydrogen atoms and one oxygen atom. The historical development of the concept of the atom is summarized in Figure 1.15 “A Summary of the Historical Development of the Concept of the Atom” .
History of Atomic Theory and the Structure of the Atom
Historical Development of Atomic Theory SlideShare
Historical Development of Atom & The Chemical Element Why Prezi. The science Conversational presenting
The History of the Atom Timeline: 400 BC Scientist: Democritus (Greek Philosopher) Democritus was a Greek philosopher who was Each atom (of an element) is different in structure from other atoms (of other elements) An atom can be divided in smaller subatomic particles: Protons, Electrons and Neutrons The nucleus is the centre of an atom. It contains protons and neutrons. Electrons orbit
3 John Dalton (1766 – 1844) father of the atomic concept. The main points of Dalton’s atomic theory were: • Elements are made of extremely small particles called atoms.
History of the Atom – Worksheet/Review sheet Name: Answer Key Period:-1. State 2 similarities between Dalton’s and the Modern Day model of the atom.
Atomic History Project Patrick County Public Schools
Molecules Chemistry Encyclopedia – structure water
The Development of Dalton’s Atomic Theory as a Case Study in the History of Science: Reflections for Educators in Chemistry He´lio Elael Bonini Viana Æ Paulo Alves Porto Published online: 4 February 2009 Springer Science Business Media B.V. 2009 Abstract The inclusion of the history of science in science curricula—and specially, in the curricula of science teachers—is a trend that has
– Historical Development Valproic acid has an analogue found in nature in the form of the valerian plant. This chemical was artificially synthesized in 1882 using the chemical in valerian as a reference. The chemical is a liquid at room temperature and was and was for a long time employed as a chemically inert solvent. Application for the drug was discovered as an anticonvulsant and
For example, water is a compound made up of 2 atoms of hydrogen and 1 atom of oxygen (a ratio of 2:1). Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water. Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water.
History leading to the creation of the atomic bomb Atomic science began many centuries ago with experimenting and probing into the nature and structure of matter. This …
History of the Atom – Worksheet/Review sheet Name: Answer Key Period:-1. State 2 similarities between Dalton’s and the Modern Day model of the atom.
Draw a diagram of an atom labeling the proton, neutron, and electron. (1 pts) (1 pts) With your group, design a poster or power point presentation of your findings.
Chemistry: Development of the Atomic Theory. STUDY. PLAY. More than 2000 years ago, Greek philosophers proposed the existence of very small, indivisible particles, each of which is called an . atom. The theory that such particles existed was supported, much later by , who proposed, in his law of , that matter can’t cannot be created or destroyed. Lavoisier Conservation of matter. Then Proposed
Historical Development Since Roman times matter had been viewed by some as discrete particles somehow linked together. Early in the eighteenth century the behavior of gases was viewed as a function of kinetic theory .
Key Historical Developments and Experiments that reveal Atoms and Atomic Structure The idea of the atom is developed: Around 400 B.C. Greek philosophers Leucippus and Democritus asserted that all material things are
2.1 Historical Development of Atomic Theory. 2 2.1.1 The Periodic Table of the Elements 2.1.2 Discovery of Subatomic Particles & the Bohr Atom Each element emits light of specific energies when excited by electric discharge or heat. For the H-atom (Balmer, 1885): 6 5 4 3 2 n = 3 2.1.2 Discovery of Subatomic Particles & the Bohr Atom Johann Jacob Balmer (Physicist, 1825 – 1899) The Hydrogen
PDF On Jan 1, 2009, Zejnilagić-Hajrić Meliha and others published Historical development of knowledge about the atom
3 John Dalton (1766 – 1844) father of the atomic concept. The main points of Dalton’s atomic theory were: • Elements are made of extremely small particles called atoms.
Abstract. The genesis and historical background of the hydro-gen bomb are described with particular emphasis placed on the development of physical ideas which led to the discovery of the
He also proposed that all of the positive charge and all of the mass of the atom occupied a small space in the center of an atom and that most of the atom is empty space occupied by electrons (Historical …
The History of the Atom Timeline: 400 BC Scientist: Democritus (Greek Philosopher) Democritus was a Greek philosopher who was Each atom (of an element) is different in structure from other atoms (of other elements) An atom can be divided in smaller subatomic particles: Protons, Electrons and Neutrons The nucleus is the centre of an atom. It contains protons and neutrons. Electrons orbit
History Of The Atom ProProfs Quiz
Historical development of the atom essays research
For example, water is a compound made up of 2 atoms of hydrogen and 1 atom of oxygen (a ratio of 2:1). Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water. Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water.
Dalton and many others continued to believe that water particles contained one hydrogen atom and one oxygen atom, rather than two hydrogen atoms and one oxygen atom. The historical development of the concept of the atom is summarized in Figure 1.15 “A Summary of the Historical Development of the Concept of the Atom” .
Historical Development Since Roman times matter had been viewed by some as discrete particles somehow linked together. Early in the eighteenth century the behavior of gases was viewed as a function of kinetic theory .
PDF On Jan 1, 2009, Zejnilagić-Hajrić Meliha and others published Historical development of knowledge about the atom
Purpose. To explore the early history of the periodic table and how it contributed to the understanding of atoms. Context. This lesson is the third of a five-part series that will broaden and enhance students’ understanding of the atom and the history of its discovery and development …
Intel Chips Throughout Intel’s history, new and improved technologies have transformed the human experience. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
He also proposed that all of the positive charge and all of the mass of the atom occupied a small space in the center of an atom and that most of the atom is empty space occupied by electrons (Historical …
history, scientists have accepted five major different atomic models. Our perception of the Our perception of the atom has changed from the early Greek model because of …
– Historical Development Valproic acid has an analogue found in nature in the form of the valerian plant. This chemical was artificially synthesized in 1882 using the chemical in valerian as a reference. The chemical is a liquid at room temperature and was and was for a long time employed as a chemically inert solvent. Application for the drug was discovered as an anticonvulsant and
historical studies has taught us about historical economic growth and development. 7.1.1 TheOriginsoftheLiterature The origins of the historical development literature can …
For example, water is a compound made up of 2 atoms of hydrogen and 1 atom of oxygen (a ratio of 2:1). Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water. Three atoms of hydrogen and 2 atoms of oxygen cannot combine to make water.
History of the Atom Bomb
Intel Chips timeline
Historical development of the atom essays research
Calculated that the atom consisted mostly of empty space through which electrons move and also concluded that there was a tiny, dense region “the nucleus” centrally located within the atom that contained all of an atom’s positive charge and virtually all of its mass.
Historical Development of Atom & The Chemical Prezi
History of the Atom Bomb
Key Historical Developments and Experiments that reveal Atoms and Atomic Structure The idea of the atom is developed: Around 400 B.C. Greek philosophers Leucippus and Democritus asserted that all material things are
Intel Chips timeline
History of the Atom – Worksheet/Review sheet Name: Answer Key Period:-1. State 2 similarities between Dalton’s and the Modern Day model of the atom.
Historical development of the atom essays research
Historical Development of the Atom Model
Dalton and many others continued to believe that water particles contained one hydrogen atom and one oxygen atom, rather than two hydrogen atoms and one oxygen atom. The historical development of the concept of the atom is summarized in Figure 1.15 “A Summary of the Historical Development of the Concept of the Atom” .
Atomic History Project Patrick County Public Schools
Molecules Chemistry Encyclopedia – structure water