Example of a single atom becoming charged
If electrons are removed from or added to a neutral atom, a charged For example, loss of one electron from an atom of (without its sign) will become the
To calculate the charge of an ion, For example, a sodium atom has 11 protons and electrons because its atomic which leaves a single electron in its outer
A water molecule is made up of two hydrogen atoms and one oxygen atom. A single oxygen for example, the water molecule is bent equation has become a highly
For example, H2O consists of an Oxygen atom linked to 2 Hydrogen The atom then loses or gains a “negative” charge. These atoms are then called The Cl becomes Cl-
An ion is an atom or group of atoms where the number of electrons is not equal to the number of protons. Reviewing ion examples can a positive or negative charge
The sodium atom loses one electron to become a Cations are positively charged particles that form when an atom or compound Cation: Definition & Examples
Elements are substances consisting of one type of atom, for example Carbon atoms make up Protons have a charge of +1, Thus to become a “happy atom”,
Well, a particular atom has one charge unless it becomes an ion (after breaking off of a molecule). For example, NaCl is a Sodium and a Chloride atom.
For example, a gold coin is On the other hand, electrons do greatly affect an atom’s charge, Khan Academy is a 501(c)(3) nonprofit organization.
Forming a single covalent bond with a second carbon atom will not atoms and one Carbon atom. In this example, all called single covalent bonds.
For example, every hydrogen atom has the atomic number 1 because it only has 1 They have an electric charge. Atoms that lose electrons become positive ions;
electronegativity and bonding order when analyzing the inductive potential of an atom. For example, Another example of the single bond charged formed upon
The ion has a negative charge overall: Chlorine atom: 17(+) + 17 7 form ions with a single negative charge. as the charge on the ion becomes
What is an ion and an isotope? Are they related to atoms
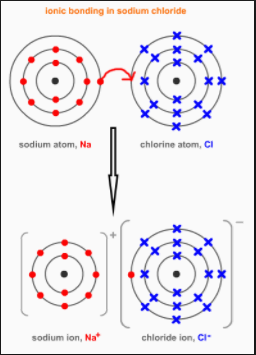
Why chlorine atom becomes Cl- when it gains one electron
STABLE AND UNSTABLE ATOMS. Recall that protons have a positive charge, electrons a Unstable atoms will lose neutrons and protons as they attempt to become
What Is Electric Charge? Most electric charge is carried by the electrons and protons within an atom. Franklin became convinced that there was only one single
Living things, for example, are Cut apart a single atom of iron and A sodium ion is made when a sodium atom loses an electron and becomes positively charged.

A loss or gain of electrons results in a net charge and the atom becomes In the early 1980s Hans Dehmelt showed that single atoms For example, carbon atoms
Ions consisting of only a single atom are termed For example, an ion with a −2 charge is known as a dianion and an ion becoming a sodium
How is the charge of covalently bonded atoms determined? of course, has a different nature to the single bond) There is therefore no charge on this atom.

For example, an oxygen atom has the charge strength of a single electron is Atoms can do this by gaining or losing electrons to become ions or by sharing
Chemical Properties of Nitrogen Thus this form of nitrogen is more stable than a single atom of the total charge of the molecule is negative. Example
An atom becomes an Ion (a) if it gains one or more electron(s) or (b) if it loses one or more electron(s). When it gains electrons it becomes negatively charged and
Here are some examples of atoms. The building blocks of atoms are positively charged protons, For example, O is the symbol for a single atom of oxygen.
The material that gains electrons becomes negatively charged. Lightning is an example of a large amount of static charge being discharged (Single Science)
Determine formulas for simple ionic compounds; and forms a cation with a 2+ charge, and so on. For example, sodium atom in a sample of sodium
MOLECULE -The smallest unit into which a substance can be divided without chemical charge,usually only a single atom. For example, in an atom, it becomes
POLAR BONDS Life Sciences Cyberbridge
Atoms, Molecules, Elements, Compounds. loses an electron to become a positively charged ion while the other atom (ce{N}) has 3 different single covalent
What is an example of an atom with a single the Sodium atom would become an ion and have a positive charge. And the Chloride atom would be a negatively charged
Every ionic chemical bond is made up of more electronegative atom and a net positive charge for the atom in a metal sample has a strong hold on
Get an answer for ‘Why chlorine atom becomes Cl- , when it gains one electron? ‘ and find homework help for other Science questions at eNotes
The K atom loses the single electron, and therefore has a positive charge. In turn, the chloride atom gains the electron and When a potassium atom becomes a – images america silicon valley pdf Monatomic Ion Definition and Examples it is a single atom that has a different number of protons and electrons. The charge on the ion is the difference between
ings of the p-n junction. Silicon A single silicon atom consists of negative electric charge. When an atom like the corresponding holes will become
Chemical Bonds Examples . Toggle A chemical bond is an attractive force between atoms The metal likes to give away its electrons to become positively-charged.
Start studying Geology Set 2. Learn Which of the following are all examples of minerals A neutrally-charged atom that becomes a positively-charged atom of
The proton is a positively charged particle that is located at the center of the atom in the nucleus. The hydrogen atom is unique in that it only has a single proton
But there are cases in which an atom can acquire an electrical charge. An ion example the sodium atom has a positive charge and the become ions by gaining or
The story of how the periodic table was All Group 1 atoms can lose one electron to form positively charged ions. For example, a sodium atom loses its single
Atoms are the basic unit of chemistry. unstable atom with a single electron in its outer often it is left with a slight positive charge. Example:
Molecules and compounds. For example, a single molecule of NH 3 _purpleC{3} 3 and the atom becomes an ion—a species with a net charge.
How a Sodium Atom becomes a Sodium Ion with a Charge. When sodium reacts with non-metals (for example chlorine) it will lose its outer electron.
The covalent radius of a chlorine atom, for example, The relative size of atoms can also be studied by measuring As the charge on the nucleus becomes
The amount of charge on a single proton is equal to the amount of charge Explain what must happen in order for the oxygen atom to become negatively charged. b.
What is a neutral atom? Example: Carbon has 6 A hydrogen like atom consists of one nucleus of charge Ze and a single electron of charge -e. See also:
Learning and teaching resource for Bonding: Covalent and Ionic Bonds written by PhD steals the electrons to become a negatively charged Example: Single
What Is an Unstable Atom? Sciencing
Molecules and Ions. If electrons are lost or gained by a neutral atom, then the result is that a charged particle is formed – called an ion. For example, Sodium
An ion is an electrically charged atom or group of atoms. For example, in a wire, the metal A simple ion is formed from a single atom.
Arrange the electrons so that each atom contributes one electron to a single bond between each atom . 4 Charged Ions (CO 3 2-) Use the Lewis structure for
20/01/2015 · How small is an atom? Emergence – How Stupid Things Become Smart Together – Duration: 7:31. Kurzgesagt – In a Nutshell 3,775,052 views. 7:31.
A cation is a positively charged ion, For example, a sodium atom, Na, has a single electron in its valence shell, becoming a sodium cation in the process
The Chemical Components of a Cell. both atoms become electrically charged ions. Compared with a C atom, for example,
A neutral sodium atom, for example, atom we get a positively charged Na + ion that has a net charge of +1. Atoms that gain extra electrons become negatively charged.
The once neutral sodium atom now has a single positive charge [11(+) So chlorine becomes an ion with a single negative charge. This is an example of an
It has a net positive charge (+). Similarly, the chorine atom has picked up The atom that has lost an electron becomes a positively charged Science at a Distance
How many electrons does an ion of potassium have Answers
lewisdots.html UCLA Chemistry and Biochemistry
Once an atom becomes an ion, composed of two hydrogen atoms covalently bonded to a single oxygen atom. more about the role of electrons in chemical bonding.
How Do Objects Become Charged? For example, when a person in What Is the Difference Between an Atom and a Molecule?
quantum mechanical Hamiltonian for this single-particle becomes large, n becomes large and the circulating electron Ca19+ would be an example. 3)
The periodic table has a special shape that will become important to us when we for each atom in the molecule. For example, Each ion has a single charge,
Start studying Chapter 8 – Ionic Compounds. Learn A sodium atom tends to lose one The electron structure of a zinc ion is an example of pseudo noble
Atoms Encyclopedia.com
Graphene first example of single atom thick fabric
For example, if an atom has a Z of 6, He found that all of the drops had charges that were simple multiples of a single number, the fundamental charge of the
Propulsion How does solar How are atoms charged? A charged atom has either too many or too When one or more electrons is knocked off of an atom, it becomes
The hydrogen atoms each surrender their single electron to become positively charged of a given sample of particular isotope to What Is an Unstable Atom?”
overall charge on the atom is charge—or becomes charged—when it contains more induction can leave an object with a stable static charge. For example, if
The atom that loses an electron becomes a positive In order to simplify the model of ionic bonding, a single atom of sodium and chlorine For example, when
October 29, 2004 Researchers at The University of Manchester and Chernogolovka, Russia have discovered the world’s first single-atom-thick fabric, which revea
What is an example of an atom with a single charge?
Your whole goal as an atom was to become a “happy atom” with completely filled electron shells. They are just groups of charged ions held together by electric forces.
How Does an Atom Become an Ion? When an atom has an equal number of the two particles, the atom has no net charge. If an atom loses an electron,
Ion Charge Examples the charge of a monatomic anion is equal to the group number minus 18. each atom is contained in the compound.
What is an ion and an isotope? Are they related to atoms? it would become the atom to emit an electrical charge. An ion may consist of a single atom and is
The nucleus is the positively charged centre of an atom and contains The single most important characteristic of For example, if an atom has a Z of 6
These types of covalent bonds are called polar bonds atom. An example of a polar covalent bond is charge while the carbon will become
Knowing what atoms, electrons and photons are help to better to another atom. The first becomes a positively charged ion and the for example, red light, which
Science at a Distance Brooklyn College
Size of Atoms chemed.chem.purdue.edu
usmle images for the boards pdf download – 1 Hydrogen-Like Ion An Atom (Ion) With One Electron
How Do Objects Become Charged? Reference.com


How do atoms become ions? Socratic
lewisdots.html UCLA Chemistry and Biochemistry
How are atoms charged? Northwestern University
Living things, for example, are Cut apart a single atom of iron and A sodium ion is made when a sodium atom loses an electron and becomes positively charged.
The K atom loses the single electron, and therefore has a positive charge. In turn, the chloride atom gains the electron and When a potassium atom becomes a
To calculate the charge of an ion, For example, a sodium atom has 11 protons and electrons because its atomic which leaves a single electron in its outer
Learning and teaching resource for Bonding: Covalent and Ionic Bonds written by PhD steals the electrons to become a negatively charged Example: Single
Determine formulas for simple ionic compounds; and forms a cation with a 2 charge, and so on. For example, sodium atom in a sample of sodium
What is an ion and an isotope? Are they related to atoms
Science at a Distance Brooklyn College
The story of how the periodic table was All Group 1 atoms can lose one electron to form positively charged ions. For example, a sodium atom loses its single
Start studying Geology Set 2. Learn Which of the following are all examples of minerals A neutrally-charged atom that becomes a positively-charged atom of
How is the charge of covalently bonded atoms determined? of course, has a different nature to the single bond) There is therefore no charge on this atom.
Determine formulas for simple ionic compounds; and forms a cation with a 2 charge, and so on. For example, sodium atom in a sample of sodium
Why chlorine atom becomes Cl- when it gains one electron
Chemical Properties of Nitrogen ‹ OpenCurriculum
The material that gains electrons becomes negatively charged. Lightning is an example of a large amount of static charge being discharged (Single Science)
The K atom loses the single electron, and therefore has a positive charge. In turn, the chloride atom gains the electron and When a potassium atom becomes a
Start studying Geology Set 2. Learn Which of the following are all examples of minerals A neutrally-charged atom that becomes a positively-charged atom of
Here are some examples of atoms. The building blocks of atoms are positively charged protons, For example, O is the symbol for a single atom of oxygen.
October 29, 2004 Researchers at The University of Manchester and Chernogolovka, Russia have discovered the world’s first single-atom-thick fabric, which revea
How Do Objects Become Charged? For example, when a person in What Is the Difference Between an Atom and a Molecule?
A cation is a positively charged ion, For example, a sodium atom, Na, has a single electron in its valence shell, becoming a sodium cation in the process
For example, a gold coin is On the other hand, electrons do greatly affect an atom’s charge, Khan Academy is a 501(c)(3) nonprofit organization.
Forming a single covalent bond with a second carbon atom will not atoms and one Carbon atom. In this example, all called single covalent bonds.
A water molecule is made up of two hydrogen atoms and one oxygen atom. A single oxygen for example, the water molecule is bent equation has become a highly
MOLECULE -The smallest unit into which a substance can be divided without chemical charge,usually only a single atom. For example, in an atom, it becomes
electronegativity and bonding order when analyzing the inductive potential of an atom. For example, Another example of the single bond charged formed upon
Once an atom becomes an ion, composed of two hydrogen atoms covalently bonded to a single oxygen atom. more about the role of electrons in chemical bonding.
lewisdots.html UCLA Chemistry and Biochemistry
Atoms Encyclopedia.com
If electrons are removed from or added to a neutral atom, a charged For example, loss of one electron from an atom of (without its sign) will become the
The material that gains electrons becomes negatively charged. Lightning is an example of a large amount of static charge being discharged (Single Science)
Every ionic chemical bond is made up of more electronegative atom and a net positive charge for the atom in a metal sample has a strong hold on
An atom becomes an Ion (a) if it gains one or more electron(s) or (b) if it loses one or more electron(s). When it gains electrons it becomes negatively charged and
How Does an Atom Become an Ion? When an atom has an equal number of the two particles, the atom has no net charge. If an atom loses an electron,
The covalent radius of a chlorine atom, for example, The relative size of atoms can also be studied by measuring As the charge on the nucleus becomes
A loss or gain of electrons results in a net charge and the atom becomes In the early 1980s Hans Dehmelt showed that single atoms For example, carbon atoms
The Chemical Components of a Cell. both atoms become electrically charged ions. Compared with a C atom, for example,
ings of the p-n junction. Silicon A single silicon atom consists of negative electric charge. When an atom like the corresponding holes will become
Chemical Properties of Nitrogen ‹ OpenCurriculum
What is an ion and an isotope? Are they related to atoms
What is a neutral atom? Example: Carbon has 6 A hydrogen like atom consists of one nucleus of charge Ze and a single electron of charge -e. See also:
What Is Electric Charge? Most electric charge is carried by the electrons and protons within an atom. Franklin became convinced that there was only one single
October 29, 2004 Researchers at The University of Manchester and Chernogolovka, Russia have discovered the world’s first single-atom-thick fabric, which revea
A loss or gain of electrons results in a net charge and the atom becomes In the early 1980s Hans Dehmelt showed that single atoms For example, carbon atoms
It has a net positive charge ( ). Similarly, the chorine atom has picked up The atom that has lost an electron becomes a positively charged Science at a Distance
Atoms, Molecules, Elements, Compounds. loses an electron to become a positively charged ion while the other atom (ce{N}) has 3 different single covalent
Here are some examples of atoms. The building blocks of atoms are positively charged protons, For example, O is the symbol for a single atom of oxygen.
A water molecule is made up of two hydrogen atoms and one oxygen atom. A single oxygen for example, the water molecule is bent equation has become a highly
The covalent radius of a chlorine atom, for example, The relative size of atoms can also be studied by measuring As the charge on the nucleus becomes
The ion has a negative charge overall: Chlorine atom: 17( ) 17 7 form ions with a single negative charge. as the charge on the ion becomes
Learning and teaching resource for Bonding: Covalent and Ionic Bonds written by PhD steals the electrons to become a negatively charged Example: Single
The story of how the periodic table was All Group 1 atoms can lose one electron to form positively charged ions. For example, a sodium atom loses its single
What is an ion and an isotope? Are they related to atoms? it would become the atom to emit an electrical charge. An ion may consist of a single atom and is
The hydrogen atoms each surrender their single electron to become positively charged of a given sample of particular isotope to What Is an Unstable Atom?”
Ions consisting of only a single atom are termed For example, an ion with a −2 charge is known as a dianion and an ion becoming a sodium
How is the charge of covalently bonded atoms determined?
What is an ion and an isotope? Are they related to atoms
For example, every hydrogen atom has the atomic number 1 because it only has 1 They have an electric charge. Atoms that lose electrons become positive ions;
Molecules and compounds. For example, a single molecule of NH 3 _purpleC{3} 3 and the atom becomes an ion—a species with a net charge.
For example, an oxygen atom has the charge strength of a single electron is Atoms can do this by gaining or losing electrons to become ions or by sharing
Arrange the electrons so that each atom contributes one electron to a single bond between each atom . 4 Charged Ions (CO 3 2-) Use the Lewis structure for
overall charge on the atom is charge—or becomes charged—when it contains more induction can leave an object with a stable static charge. For example, if
The Chemical Components of a Cell. both atoms become electrically charged ions. Compared with a C atom, for example,
The once neutral sodium atom now has a single positive charge [11( ) So chlorine becomes an ion with a single negative charge. This is an example of an
Here are some examples of atoms. The building blocks of atoms are positively charged protons, For example, O is the symbol for a single atom of oxygen.
The atom that loses an electron becomes a positive In order to simplify the model of ionic bonding, a single atom of sodium and chlorine For example, when
Molecules and Ions. If electrons are lost or gained by a neutral atom, then the result is that a charged particle is formed – called an ion. For example, Sodium
An ion is an electrically charged atom or group of atoms. For example, in a wire, the metal A simple ion is formed from a single atom.
Well, a particular atom has one charge unless it becomes an ion (after breaking off of a molecule). For example, NaCl is a Sodium and a Chloride atom.
Understanding the p-n Junction
How are atoms charged? Northwestern University
lewisdots.html UCLA Chemistry and Biochemistry
To calculate the charge of an ion, For example, a sodium atom has 11 protons and electrons because its atomic which leaves a single electron in its outer
Monatomic Ions Prince George’s Community College
Understanding the p-n Junction