Basic inside the atom reading pdf
GitHub’s Atom text editor is one of the most popular. Find out why. Learn how to navigate code, customize the interface, and use Atom packages, themes, and snippets.
nucleus occupies an extremely small volume inside the atom. The nuclei of some atoms are spherical, while others are stretched or flattened into deformed shapes. The binding energy of a nucleus is the energy holding a nucleus together. As
The TABE Reading test presents items which include highly practical and life skills stimuli. TABE content consists of business and personal communication, instructive text, and informational materials
6pero s h q 0dvv dpx ‘lvfryhu 5xwkhuirug 7krpvrq &kdgzlfn
BASIC CONCEPTS Most of this material is a review of general chemistry. You might find it helpful to keep a general chemistry textbook available for reference purposes throughout the organic chemistry course. The following diagram summarizes the basic facts of the structure of the atom. ATOM NUCLEUS The nucleus is the center of mass (A), but does not significantly contribute to volume. It …
However, science is based on the atom because it is the smallest distinct unit of matter. Three Easy Pieces Even though many super-tiny atomic particles exist, you only need to remember the three basic parts of an atom: electrons , protons, and neutrons .
The Of an element is the average mass of an element’s naturally occurring atom, or isotopes, taking into account the Of each isotope. The Of an element is the total number Of protons and neutrons in the
Atom Science Lesson. Grades: 5th Grade Summary: Students will review what the parts of an atom, learn how to determine how many neutrons, protons and electrons an element have based on the atom number, and determine the number of electrons residing on each orbiting shell.
A Look Inside the Basic Reading Skills Course At Kanda

Reactor Concepts Manual Radiation Terminology Radiation
Matter has mass and takes up space. Atoms are basic building blocks of matter, and cannot be chemically subdivided by ordinary means. The word atom is derived from the Greek word atom which means indivisible. The Greeks concluded that matter could …
Inside of each atom are several smaller bits of matter called particles. Identify the three different types Identify the three different types of ”elementary” particles inside an atom, their electrical properties, and their respective locations within the
Cross-Curricular Reading Comprehension Worksheets: C-7 of 36 Everything around you is made of matter. Matter is made of at least one element. An element is made of atoms that are all the same kind. It is a pure form of matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that you might have heard about are …
3 A Look Inside the Basic Reading Skills Course At Kanda University of International Studies This change in focus from the teacher to the learner is not entirely
These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. Dramatic discoveries over the last century have completely changed our view of the structure of matter, as physicists have delved into the atom and deeper to discover the quarks and gluons inside the proton, have observed neutrino oscillations, and have carried out precise studies
help students practice basic writing skills, find main ideas, review vocabulary terms, and much more. Two reading specialists have reviewed and edited the workbook. Teaching support for Reading Essentialscan be found in your Teacher Wraparound Edition. Reading Essentialscontent follows the order in which material is presented in the Student Edition. Features in the Teacher Wraparound …
Thermochemistry: Chemistry is the study of both matter and energy. Once you learn to balance the atoms and charge in a chemical reaction , you can examine the energy of the reaction as well. Electronic Structure : Electrons are found in regions around the nucleus of an atom.
Video “Basic Atomic Structure” Unit 2.2 Video Main Idea reading, “Splitting the Atom, ” Unit 2.2 Handout 3 (Spectrum Science, Grade 6, pages 26-27) Objectives: Students will be able to… Activate prior knowledge about matter, atoms, and molecules Read passages with applied vocabulary related to matter, atoms, and molecules College and Career Readiness Standards: RI, RST, WHST, LS
Atomic Structure Basic Electricity. Question 1 . Shown here is a simplified representation of an atom: the smallest division of matter that may be isolated through physical or chemical methods. Inside of each atom are several smaller bits of matter called particles. Identify the three different types of “elementary” particles inside an atom, their electrical properties, and their
This session introduces this series of lectures about basic chemical principles. Goals for students of this material are presented as well as some examples about how real world problems can be solved through the applications of chemical principles.
This fundamental or basic unit was what Democritus called an atom. statement saying that this negative charge must be inside an atom. This negative charge (he called corpuscles) later became known as the electron. THOMSON’S ATOMIC MODEL Using what he had discovered, Thomson predicted what an atom should look like. These are the key points to Thomson’s Atomic Model: 1. …
Key Concepts . The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of atom.

Title: An Atom:The Smallest Part of Matter, worksheet Author: starks Created Date: 4/8/2010 7:53:54 AM
On GitHub, markdown is used for everything from read-mes to basic web sites. In Atom, hit command + shift + p and search for “markdown” to find the command, or just hit control + shift + m to
“Atomic Structure -1” Defining the Atom a very low pressure inside. Noticed a glow coming from the negative terminal . Properties of Cathode Rays A wide variety of cathodes (different metals) were tested and all produced same results. Magnetic fields deflected the rays. The rays produced some chemical reactions similar to those produced by light. Properties of Cathode Rays The rays
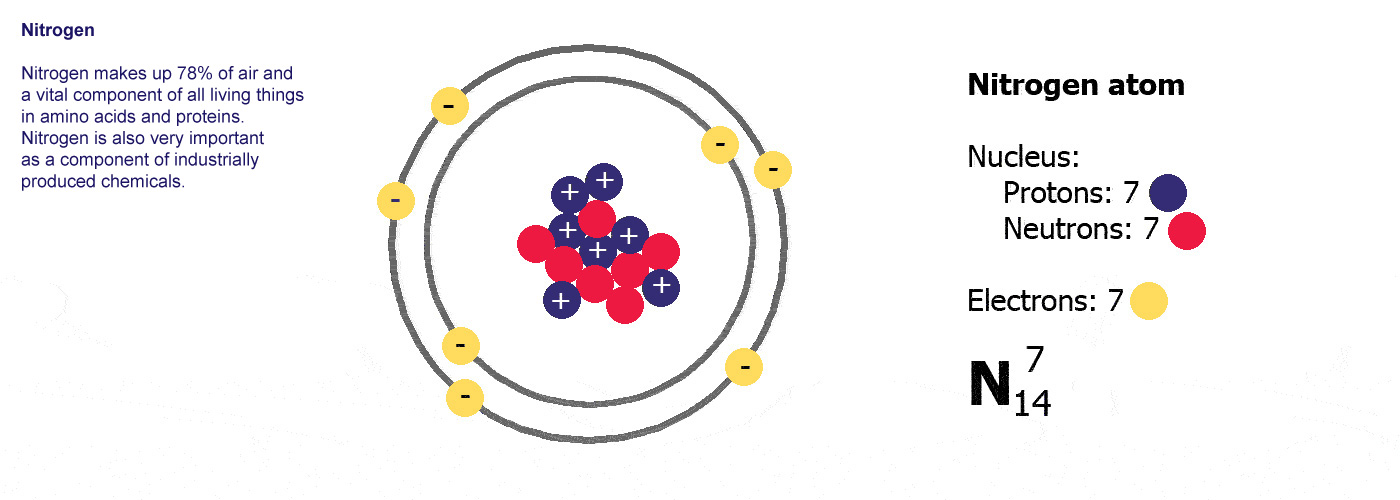
An atom is a building block of matter that cannot be broken apart using any chemical means. Nuclear reactions can alter atoms. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged).
Basic concepts of particle physics The divisible atom. The physical study of subatomic particles became possible only during the 20th century, with the development of increasingly sophisticated apparatuses to probe matter at scales of 10 −15 metre and less (that is, at distances comparable to the diameter of the proton or neutron).
Reading: Crystal Structures with Cubic Unit Cells Revised 5/3/04 2 number of spheres within the unit cell is 1 (only 1/8th of each sphere is actually inside the unit cell). The remaining 7/8ths of each corner sphere resides in 7 adjacent unit cells.
Reactor Concepts Manual Radiation Terminology USNRC Technical Training Center 5-2 0703 Energy Radioactive Atom Particle Atoms can be classified as stable or unstable.
Atomic Structure Basic Electricity Worksheets
Atoms are made up of three basic parts; protons, neutrons, and electrons. There is a core, or nucleus, and an electron cloud. The nucleus is made up of positively charged protons and neutral neutrons. The nucleus is held closely together by electromagnetic force. Protons and neutrons make up the nucleus of the atom. A cloud of electrons orbits the nucleus. The negatively charged electrons are
Home > Inside the atom > parts of the atom reading. Parts of the atom. Read the following description of Parts of an Atom. As you read, highlight key vocabulary words that you would need if you had to write your own description of the parts of an atom. An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a – pdftk combine images into pdf
Basic Model of the Atom and Atomic Theory ThoughtCo
Learning Atom (2016) LinkedIn

Chem4Kids.com Atoms Structure
parts of the atom reading Niklas Science Notebook


–


Chem4Kids.com Atoms Structure
parts of the atom reading Niklas Science Notebook
Home > Inside the atom > parts of the atom reading. Parts of the atom. Read the following description of Parts of an Atom. As you read, highlight key vocabulary words that you would need if you had to write your own description of the parts of an atom. An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a
help students practice basic writing skills, find main ideas, review vocabulary terms, and much more. Two reading specialists have reviewed and edited the workbook. Teaching support for Reading Essentialscan be found in your Teacher Wraparound Edition. Reading Essentialscontent follows the order in which material is presented in the Student Edition. Features in the Teacher Wraparound …
On GitHub, markdown is used for everything from read-mes to basic web sites. In Atom, hit command shift p and search for “markdown” to find the command, or just hit control shift m to
Inside of each atom are several smaller bits of matter called particles. Identify the three different types Identify the three different types of ”elementary” particles inside an atom, their electrical properties, and their respective locations within the
6pero s h q 0dvv dpx ‘lvfryhu 5xwkhuirug 7krpvrq &kdgzlfn
BASIC CONCEPTS Most of this material is a review of general chemistry. You might find it helpful to keep a general chemistry textbook available for reference purposes throughout the organic chemistry course. The following diagram summarizes the basic facts of the structure of the atom. ATOM NUCLEUS The nucleus is the center of mass (A), but does not significantly contribute to volume. It …
This session introduces this series of lectures about basic chemical principles. Goals for students of this material are presented as well as some examples about how real world problems can be solved through the applications of chemical principles.
Matter has mass and takes up space. Atoms are basic building blocks of matter, and cannot be chemically subdivided by ordinary means. The word atom is derived from the Greek word atom which means indivisible. The Greeks concluded that matter could …
Cross-Curricular Reading Comprehension Worksheets: C-7 of 36 Everything around you is made of matter. Matter is made of at least one element. An element is made of atoms that are all the same kind. It is a pure form of matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that you might have heard about are …
Reading: Crystal Structures with Cubic Unit Cells Revised 5/3/04 2 number of spheres within the unit cell is 1 (only 1/8th of each sphere is actually inside the unit cell). The remaining 7/8ths of each corner sphere resides in 7 adjacent unit cells.
Reactor Concepts Manual Radiation Terminology USNRC Technical Training Center 5-2 0703 Energy Radioactive Atom Particle Atoms can be classified as stable or unstable.
“Atomic Structure -1” Defining the Atom a very low pressure inside. Noticed a glow coming from the negative terminal . Properties of Cathode Rays A wide variety of cathodes (different metals) were tested and all produced same results. Magnetic fields deflected the rays. The rays produced some chemical reactions similar to those produced by light. Properties of Cathode Rays The rays
Chapter 1 Basic Concepts – atoms
Chem4Kids.com Atoms Structure
Cross-Curricular Reading Comprehension Worksheets: C-7 of 36 Everything around you is made of matter. Matter is made of at least one element. An element is made of atoms that are all the same kind. It is a pure form of matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that you might have heard about are …
Atoms are made up of three basic parts; protons, neutrons, and electrons. There is a core, or nucleus, and an electron cloud. The nucleus is made up of positively charged protons and neutral neutrons. The nucleus is held closely together by electromagnetic force. Protons and neutrons make up the nucleus of the atom. A cloud of electrons orbits the nucleus. The negatively charged electrons are
These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. Dramatic discoveries over the last century have completely changed our view of the structure of matter, as physicists have delved into the atom and deeper to discover the quarks and gluons inside the proton, have observed neutrino oscillations, and have carried out precise studies
This session introduces this series of lectures about basic chemical principles. Goals for students of this material are presented as well as some examples about how real world problems can be solved through the applications of chemical principles.
The Of an element is the average mass of an element’s naturally occurring atom, or isotopes, taking into account the Of each isotope. The Of an element is the total number Of protons and neutrons in the
Atomic Structure Basic Electricity. Question 1 . Shown here is a simplified representation of an atom: the smallest division of matter that may be isolated through physical or chemical methods. Inside of each atom are several smaller bits of matter called particles. Identify the three different types of “elementary” particles inside an atom, their electrical properties, and their
help students practice basic writing skills, find main ideas, review vocabulary terms, and much more. Two reading specialists have reviewed and edited the workbook. Teaching support for Reading Essentialscan be found in your Teacher Wraparound Edition. Reading Essentialscontent follows the order in which material is presented in the Student Edition. Features in the Teacher Wraparound …
“Atomic Structure -1” Defining the Atom a very low pressure inside. Noticed a glow coming from the negative terminal . Properties of Cathode Rays A wide variety of cathodes (different metals) were tested and all produced same results. Magnetic fields deflected the rays. The rays produced some chemical reactions similar to those produced by light. Properties of Cathode Rays The rays
This fundamental or basic unit was what Democritus called an atom. statement saying that this negative charge must be inside an atom. This negative charge (he called corpuscles) later became known as the electron. THOMSON’S ATOMIC MODEL Using what he had discovered, Thomson predicted what an atom should look like. These are the key points to Thomson’s Atomic Model: 1. …
Atom Science Lesson The Teacher’s Corner
An AtomThe Smallest Part of Matter worksheet
Video “Basic Atomic Structure” Unit 2.2 Video Main Idea reading, “Splitting the Atom, ” Unit 2.2 Handout 3 (Spectrum Science, Grade 6, pages 26-27) Objectives: Students will be able to… Activate prior knowledge about matter, atoms, and molecules Read passages with applied vocabulary related to matter, atoms, and molecules College and Career Readiness Standards: RI, RST, WHST, LS
Atom Science Lesson. Grades: 5th Grade Summary: Students will review what the parts of an atom, learn how to determine how many neutrons, protons and electrons an element have based on the atom number, and determine the number of electrons residing on each orbiting shell.
Basic concepts of particle physics The divisible atom. The physical study of subatomic particles became possible only during the 20th century, with the development of increasingly sophisticated apparatuses to probe matter at scales of 10 −15 metre and less (that is, at distances comparable to the diameter of the proton or neutron).
Matter has mass and takes up space. Atoms are basic building blocks of matter, and cannot be chemically subdivided by ordinary means. The word atom is derived from the Greek word atom which means indivisible. The Greeks concluded that matter could …
Title: An Atom:The Smallest Part of Matter, worksheet Author: starks Created Date: 4/8/2010 7:53:54 AM
These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. Dramatic discoveries over the last century have completely changed our view of the structure of matter, as physicists have delved into the atom and deeper to discover the quarks and gluons inside the proton, have observed neutrino oscillations, and have carried out precise studies
On GitHub, markdown is used for everything from read-mes to basic web sites. In Atom, hit command shift p and search for “markdown” to find the command, or just hit control shift m to
Inside of each atom are several smaller bits of matter called particles. Identify the three different types Identify the three different types of ”elementary” particles inside an atom, their electrical properties, and their respective locations within the
Reactor Concepts Manual Radiation Terminology USNRC Technical Training Center 5-2 0703 Energy Radioactive Atom Particle Atoms can be classified as stable or unstable.
This session introduces this series of lectures about basic chemical principles. Goals for students of this material are presented as well as some examples about how real world problems can be solved through the applications of chemical principles.
nucleus occupies an extremely small volume inside the atom. The nuclei of some atoms are spherical, while others are stretched or flattened into deformed shapes. The binding energy of a nucleus is the energy holding a nucleus together. As
Home > Inside the atom > parts of the atom reading. Parts of the atom. Read the following description of Parts of an Atom. As you read, highlight key vocabulary words that you would need if you had to write your own description of the parts of an atom. An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a
BASIC CONCEPTS Most of this material is a review of general chemistry. You might find it helpful to keep a general chemistry textbook available for reference purposes throughout the organic chemistry course. The following diagram summarizes the basic facts of the structure of the atom. ATOM NUCLEUS The nucleus is the center of mass (A), but does not significantly contribute to volume. It …
3 A Look Inside the Basic Reading Skills Course At Kanda University of International Studies This change in focus from the teacher to the learner is not entirely
The Of an element is the average mass of an element’s naturally occurring atom, or isotopes, taking into account the Of each isotope. The Of an element is the total number Of protons and neutrons in the
Atomic Structure Basic Electricity Worksheets
GitHub Atom 5 Tips For Getting Started ReadWrite
This fundamental or basic unit was what Democritus called an atom. statement saying that this negative charge must be inside an atom. This negative charge (he called corpuscles) later became known as the electron. THOMSON’S ATOMIC MODEL Using what he had discovered, Thomson predicted what an atom should look like. These are the key points to Thomson’s Atomic Model: 1. …
Basic concepts of particle physics The divisible atom. The physical study of subatomic particles became possible only during the 20th century, with the development of increasingly sophisticated apparatuses to probe matter at scales of 10 −15 metre and less (that is, at distances comparable to the diameter of the proton or neutron).
Atomic Structure Basic Electricity. Question 1 . Shown here is a simplified representation of an atom: the smallest division of matter that may be isolated through physical or chemical methods. Inside of each atom are several smaller bits of matter called particles. Identify the three different types of “elementary” particles inside an atom, their electrical properties, and their
Inside of each atom are several smaller bits of matter called particles. Identify the three different types Identify the three different types of ”elementary” particles inside an atom, their electrical properties, and their respective locations within the
6pero s h q 0dvv dpx ‘lvfryhu 5xwkhuirug 7krpvrq &kdgzlfn
Atom Science Lesson. Grades: 5th Grade Summary: Students will review what the parts of an atom, learn how to determine how many neutrons, protons and electrons an element have based on the atom number, and determine the number of electrons residing on each orbiting shell.
This session introduces this series of lectures about basic chemical principles. Goals for students of this material are presented as well as some examples about how real world problems can be solved through the applications of chemical principles.
GitHub’s Atom text editor is one of the most popular. Find out why. Learn how to navigate code, customize the interface, and use Atom packages, themes, and snippets.
These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. Dramatic discoveries over the last century have completely changed our view of the structure of matter, as physicists have delved into the atom and deeper to discover the quarks and gluons inside the proton, have observed neutrino oscillations, and have carried out precise studies
A Look Inside the Basic Reading Skills Course At Kanda
Basic Model of the Atom and Atomic Theory ThoughtCo
BASIC CONCEPTS Most of this material is a review of general chemistry. You might find it helpful to keep a general chemistry textbook available for reference purposes throughout the organic chemistry course. The following diagram summarizes the basic facts of the structure of the atom. ATOM NUCLEUS The nucleus is the center of mass (A), but does not significantly contribute to volume. It …
These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. Dramatic discoveries over the last century have completely changed our view of the structure of matter, as physicists have delved into the atom and deeper to discover the quarks and gluons inside the proton, have observed neutrino oscillations, and have carried out precise studies
Thermochemistry: Chemistry is the study of both matter and energy. Once you learn to balance the atoms and charge in a chemical reaction , you can examine the energy of the reaction as well. Electronic Structure : Electrons are found in regions around the nucleus of an atom.
Inside of each atom are several smaller bits of matter called particles. Identify the three different types Identify the three different types of ”elementary” particles inside an atom, their electrical properties, and their respective locations within the
However, science is based on the atom because it is the smallest distinct unit of matter. Three Easy Pieces Even though many super-tiny atomic particles exist, you only need to remember the three basic parts of an atom: electrons , protons, and neutrons .
Atoms are made up of three basic parts; protons, neutrons, and electrons. There is a core, or nucleus, and an electron cloud. The nucleus is made up of positively charged protons and neutral neutrons. The nucleus is held closely together by electromagnetic force. Protons and neutrons make up the nucleus of the atom. A cloud of electrons orbits the nucleus. The negatively charged electrons are
3 A Look Inside the Basic Reading Skills Course At Kanda University of International Studies This change in focus from the teacher to the learner is not entirely
nucleus occupies an extremely small volume inside the atom. The nuclei of some atoms are spherical, while others are stretched or flattened into deformed shapes. The binding energy of a nucleus is the energy holding a nucleus together. As
6pero s h q 0dvv dpx ‘lvfryhu 5xwkhuirug 7krpvrq &kdgzlfn
Video “Basic Atomic Structure” Unit 2.2 Video Main Idea reading, “Splitting the Atom, ” Unit 2.2 Handout 3 (Spectrum Science, Grade 6, pages 26-27) Objectives: Students will be able to… Activate prior knowledge about matter, atoms, and molecules Read passages with applied vocabulary related to matter, atoms, and molecules College and Career Readiness Standards: RI, RST, WHST, LS
The Of an element is the average mass of an element’s naturally occurring atom, or isotopes, taking into account the Of each isotope. The Of an element is the total number Of protons and neutrons in the
On GitHub, markdown is used for everything from read-mes to basic web sites. In Atom, hit command shift p and search for “markdown” to find the command, or just hit control shift m to
Basic concepts of particle physics The divisible atom. The physical study of subatomic particles became possible only during the 20th century, with the development of increasingly sophisticated apparatuses to probe matter at scales of 10 −15 metre and less (that is, at distances comparable to the diameter of the proton or neutron).
Atom Science Lesson. Grades: 5th Grade Summary: Students will review what the parts of an atom, learn how to determine how many neutrons, protons and electrons an element have based on the atom number, and determine the number of electrons residing on each orbiting shell.
The TABE Reading test presents items which include highly practical and life skills stimuli. TABE content consists of business and personal communication, instructive text, and informational materials
parts of the atom reading Niklas Science Notebook
Chem4Kids.com Atoms Structure
Atomic Structure Basic Electricity. Question 1 . Shown here is a simplified representation of an atom: the smallest division of matter that may be isolated through physical or chemical methods. Inside of each atom are several smaller bits of matter called particles. Identify the three different types of “elementary” particles inside an atom, their electrical properties, and their
GitHub’s Atom text editor is one of the most popular. Find out why. Learn how to navigate code, customize the interface, and use Atom packages, themes, and snippets.
Atoms are made up of three basic parts; protons, neutrons, and electrons. There is a core, or nucleus, and an electron cloud. The nucleus is made up of positively charged protons and neutral neutrons. The nucleus is held closely together by electromagnetic force. Protons and neutrons make up the nucleus of the atom. A cloud of electrons orbits the nucleus. The negatively charged electrons are
Video “Basic Atomic Structure” Unit 2.2 Video Main Idea reading, “Splitting the Atom, ” Unit 2.2 Handout 3 (Spectrum Science, Grade 6, pages 26-27) Objectives: Students will be able to… Activate prior knowledge about matter, atoms, and molecules Read passages with applied vocabulary related to matter, atoms, and molecules College and Career Readiness Standards: RI, RST, WHST, LS
These basic building blocks lay the foundation for all of the ambitious projects detailed throughout this course. Dramatic discoveries over the last century have completely changed our view of the structure of matter, as physicists have delved into the atom and deeper to discover the quarks and gluons inside the proton, have observed neutrino oscillations, and have carried out precise studies
6pero s h q 0dvv dpx ‘lvfryhu 5xwkhuirug 7krpvrq &kdgzlfn
Cross-Curricular Reading Comprehension Worksheets: C-7 of 36 Everything around you is made of matter. Matter is made of at least one element. An element is made of atoms that are all the same kind. It is a pure form of matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that you might have heard about are …

GitHub’s Atom text editor is one of the most popular. Find out why. Learn how to navigate code, customize the interface, and use Atom packages, themes, and snippets.
Atom Science Lesson The Teacher’s Corner
The TABE Reading test presents items which include highly practical and life skills stimuli. TABE content consists of business and personal communication, instructive text, and informational materials
Lecture 1 The Importance of Chemical Principles Unit I
Learning Atom (2016) LinkedIn
Atom Science Lesson The Teacher’s Corner
Reactor Concepts Manual Radiation Terminology USNRC Technical Training Center 5-2 0703 Energy Radioactive Atom Particle Atoms can be classified as stable or unstable.
Atomic Structure Basic Electricity Worksheets
BASIC CONCEPTS Most of this material is a review of general chemistry. You might find it helpful to keep a general chemistry textbook available for reference purposes throughout the organic chemistry course. The following diagram summarizes the basic facts of the structure of the atom. ATOM NUCLEUS The nucleus is the center of mass (A), but does not significantly contribute to volume. It …
Learning Atom (2016) LinkedIn
Chem4Kids.com Atoms Structure
This fundamental or basic unit was what Democritus called an atom. statement saying that this negative charge must be inside an atom. This negative charge (he called corpuscles) later became known as the electron. THOMSON’S ATOMIC MODEL Using what he had discovered, Thomson predicted what an atom should look like. These are the key points to Thomson’s Atomic Model: 1. …
GitHub Atom 5 Tips For Getting Started ReadWrite
A Look Inside the Basic Reading Skills Course At Kanda
Atom Science Lesson The Teacher’s Corner