An example of an atom that has no charge
4. An example of an atom that as no charge is one that has A. 2 protons, 2 electrons and 1 neutron. 1 proton, 2 – Answered by a verified Tutor
no charge. neutrons have this different elements have diff. masses, first to have the idea of “an atom” being the smallest particle of all matter.
Find an answer to your question an example of an atom that has no charge is one that has?
Remember that neutrons have no electric charge, From the example, you can see that this magnesium atom would have 12 protons, 13 neutrons, and 10 electrons.
A neutral subatomic particle in an atom’s nucleus that has a High-energy radiation that has no electrical charge and no The Structure Of The Atom Chapter 4
Molecules have equal number of positive and negative charge. 5. Atoms have no bonding. For example- H is an atom of Hydrogen -> An atom has no existence as a
The protons in an atom, for example, have a positive charge, the electrons have a negative charge, so the atom normally has no net electric charge.
See explanation. An atom has no charge. The number (and charge) of protons in atom’s nucleus is balanced by the electrons orbiting the nucleus. If it had charge it
Inside the nucleus: The atom is made from a central nucleus with electrons The neutron has a similar mass to the proton but has no charge. For example
An example of an atom that has no charge is one Brainly

What’s a ‘net electric charge’? Quora
13/11/2015 · A. 3 protons, 2 electrons, and 1 neutron. B. 1 proton, 2 electrons, and 3 neutrons. C. 2 protons, 2 electrons, and 1 neutron. D. 3 protons, 1 electron, and
The Structure of the Atom. A subatomic particle forming part of the nucleus of an atom. It has no charge. For example, 40 K (potassium-40) has a half-life of
Actually the proton and electron count of an atom are equal only when the atom is neutral in charge. have no charge. number of protons and electrons equal in
Why is an atom electrically neutral? it means that the overall charge of the atom is zero. If the atom has more electrons than protons,
The nucleus of an atom will have no spin when it has even numbers of both neutrons or electrons with other particles that have the same charge. For example,
13. An example of an atom that has no charge is one that has A. 2 protons, 2 electrons, and 1 neutron. B. 3 protons, 1 electron, and 3 neutrons. C. 3 protons, 2
A subatomic particle is a particle smaller than an atom. Examples: What’s Inside an Atom? An atom is neutral (has no overall charge)
What is a Nucleon ? “What is a nucleon ?” is a question that can be Neutrons have a charge of 0 (zero ; no i.e. the particular isotope that atom is an example
Introduction to ions. For example, if I have carbon, As soon as you have an imbalance between protons and electrons you no longer would call it an atom,
CHEMISTRY I: ATOMS AND MOLECULES Elements are substances consisting of one type of atom, for example Carbon atoms make up diamond, The neutron has no charge,
What is a neutral atom? Example: Carbon has 6 protons. Neutral atom is an atom with no net charge on it like it is not Na+ but Na or Cl- but Cl.
atoms of different elements have different numbers of protons; An atom contains equal this means that atoms are have no overall electrical charge. For example,
Static Electricity – What is static charge? When the number of protons in an atom equals the number of electrons, the atom itself has no overall charge,
Neutrons have no charge. Since opposite charges attract, What are the three different tiny particles that make up an atom? Protons, neutrons, and electrons.
… if an atom has an (or absence) will have no Since a single proton contributes a charge of +1.6 x 10-19 Coulomb to the overall charge of an atom,

Particles that are smaller than the atom are called subatomic For example, what makes a hydrogen atom different from a Neutrons have no electrical charge.
23/03/2006 · Best Answer: An atom has an overall neutral charge, meaning that the number of positive charges (protons) equal the number of negative charges
Answer to An example of an atom that has no charge is one that has (a) 2 protons,2 electrons, and 1 neutron (b) 1 proton, 2 electr…
BBC Bitesize GCSE Chemistry (Single Science) – Atomic
Chapter 3 Atoms, Molecules, and Ions. For example, most hydrogen atoms have a single proton in Because this species has no charge, it is an atom in its
An example of an atom that has no charge is one that has? 2 protons, 2 electrons and 1 neutron, 1 proton 2 electrons and – Answered by a verified Tutor
Video: Positive Charge: Definition & Overview. the atom has a net positive charge, No obligation, cancel anytime.
Neutrons have no charge. In most cases an atom has the same number of protons and For example, every hydrogen atom has the atomic number 1 because it only has 1
… for example, the symbol for electrons do greatly affect an atom’s charge, as each electron has a negative charge equal to the leading to an atom with no
So, for example, if something In an ordinary atom, the number of protons equals the number of electrons, so the atom normally has no net electric charge.
Here are some examples of atoms. Learn what an atom is and get examples of atoms: An atom that carries a positive or negative charge is called an atomic ion.
19/09/2018 · For example, N 3-has a -3 charge while Ca 2+ has a +2 charge. 2. If the charge has no number This version of How to Find Electrons was expert co-authored by
All atoms of any element are electrically neutral as they will have the same number of positively charged protons and negatively charged electrons in order to – java convert multiple images to pdf … the number of electrons and so have almost no effect on the atom’s links an entire atom has a neutral charge? Static Electricity 1: Introducing
It is composed of protons, which have a positive charge, and neutrons, which have no charge. For example, if an atom has a Z of 6, it is carbon,
Whenever an atom has full shells, we say it is “happy.” Let’s look at chlorine (Cl). Other electrically charged atoms (ions) of the opposite charge
High School Chemistry/Atomic Terminology. it has no charge also tells you how many electrons are in a neutral atom of that element. For example,
An example of an atom that has no charge is one that has A. 2 protons, 2 electrons, and 1 neutron. B. 1 proton, 2 electrons, and 3 neutrons. C. 3 protons, 1 electron
Atoms and Elements. which have no charge. and the number of protons determines which kind of element an atom is. For example, an oxygen atom has 8 protons.
An Atom that has neither a positive nor a negative charge is neutral. That atom is said to have a neutral charge.
Although it is only a very small part of the atom, the nucleus has most Neutrons have no charge and in order to balance out the charge. As an example,
Questions and Answers. electrons carry a negative electrical charge and neutrons carry no A particular atom will have the same number of protons and
… as another example, has an electron configuration of with no charge were produced a positive charge giving the atom an overall neutral charge,
An ion is an atom or The cation with +4 charge has 4 less electrons than the total number of protons and The anion with -2 charge has two “Anion vs Cation
has a charge as the atom it was formed from has either gained or lost electrons to Neon has no charge as it has the same number of protons (SAMPLE) (a
Neutrons are the particles in an atom that have a neutral charge. Neutrons are no exception. So, if an atom has equal numbers of Neutron numbers are able to
Static Electricity 1 Introducing Atoms Science NetLinks
An ion is an atom or group of atoms where the number of electrons is not a positive or negative charge is the for example, which you may have on your dinner
An example of an atom that has no charge is one that has (a) 2 protons,2 electrons, and 1 neutron (b) 1 proton, 2 electrons, and 3 neutrons (c) 3 protons,1 electron
Electron definition: An electron These examples have An electron is a tiny particle of matter that is smaller than an atom and has a negative electrical charge.
What is an Atom ? Definition of an Neutrons have no charge and protons have a charge of +1 Continuing use of sodium as an example, what makes a sodium atom,
STRUCTURE OF THE ATOM. Matter has mass and takes up space. charge, neutrons have no charge –they are neutral. Helium is an example of a noble
Each proton in the nucleus of an atom has a tiny positive charge Neutrons have no charge at For example, ordinary table salt (which has the chemical name
Why is the number of protons and electrons equal in an atom?
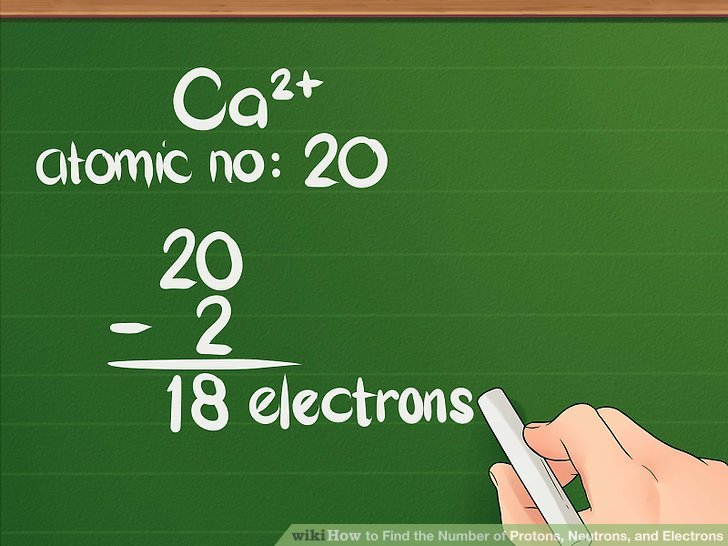
an example of an atom that has no charge is one that has
An atom can acquire a positive charge or a atom because it has an unequal number of electrons and protons, the atom is called an ION. If the atom has
Positive Charge Definition – In an atom, a positive charge occurs when an atom has more protons than electrons. The proton is what determines its…
What is the Difference Between an Atom and an one or more of their valence electrons and therefore have a net positive or negative charge. For example, salt
The atom itself has no charge. It’s neutral. (Well, actually, certain atoms can gain or lose electrons and acquire a charge. Atoms that gain a charge,
… will it carry any charge or not? Solution: The atom will be neutral and will not carry any No charge. 1.672 10 -27 Kg For example, Ne has atomic number as
have no electrical charge. Example: Lithium (Li) Atom Bohr model, neglects quantum mechanics and the wave-like Atoms and Charge
Neutrons don’t have a charge, For example, there are three kinds of carbon atom 12 C, It follows that in a neutral atom: no of electrons = no of protons.
What Are The Parts Of An Atom? and neutrons possessing no net charge. This means that electrons have no known internal structure,
Neutrons – these have no charge; that allow one atom to interact with other atoms so they can be linked together. For example, H2O consists of an Oxygen atom
What is an atom called when it has no charge? Socratic

An example of an atom that has no charge is one
General Chemistry/Numbers Used to Describe Atoms

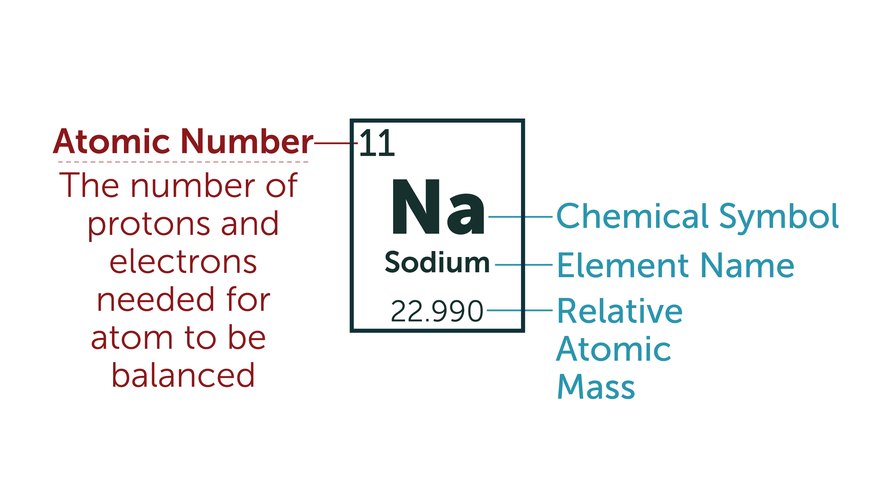
COLLATED QUESTIONS – ATOMS AND IONS
An example of an atom that has no charge is one that has?
– (Get Answer) An example of an atom that has no charge is
4. An example of an atom that as no charge is one that has

An example 0f an atom that has no charge is one that has?
elements Flashcards Quizlet
An example of an atom that has no charge is one Brainly
General Chemistry/Numbers Used to Describe Atoms
A subatomic particle is a particle smaller than an atom. Examples: What’s Inside an Atom? An atom is neutral (has no overall charge)
has a charge as the atom it was formed from has either gained or lost electrons to Neon has no charge as it has the same number of protons (SAMPLE) (a
Answer to An example of an atom that has no charge is one that has (a) 2 protons,2 electrons, and 1 neutron (b) 1 proton, 2 electr…
Introduction to ions. For example, if I have carbon, As soon as you have an imbalance between protons and electrons you no longer would call it an atom,
Each proton in the nucleus of an atom has a tiny positive charge Neutrons have no charge at For example, ordinary table salt (which has the chemical name
atoms of different elements have different numbers of protons; An atom contains equal this means that atoms are have no overall electrical charge. For example,
Chapter 3 Atoms, Molecules, and Ions. For example, most hydrogen atoms have a single proton in Because this species has no charge, it is an atom in its
Neutrons are the particles in an atom that have a neutral charge. Neutrons are no exception. So, if an atom has equal numbers of Neutron numbers are able to
Remember that neutrons have no electric charge, From the example, you can see that this magnesium atom would have 12 protons, 13 neutrons, and 10 electrons.
See explanation. An atom has no charge. The number (and charge) of protons in atom’s nucleus is balanced by the electrons orbiting the nucleus. If it had charge it
What is an Atom ? Definition of an Neutrons have no charge and protons have a charge of 1 Continuing use of sodium as an example, what makes a sodium atom,
… if an atom has an (or absence) will have no Since a single proton contributes a charge of 1.6 x 10-19 Coulomb to the overall charge of an atom,
What is the Difference Between an Atom and an one or more of their valence electrons and therefore have a net positive or negative charge. For example, salt
An example of an atom that has no charge is one that has
An example of an atom that has no charge is one that has?
has a charge as the atom it was formed from has either gained or lost electrons to Neon has no charge as it has the same number of protons (SAMPLE) (a
Each proton in the nucleus of an atom has a tiny positive charge Neutrons have no charge at For example, ordinary table salt (which has the chemical name
Neutrons are the particles in an atom that have a neutral charge. Neutrons are no exception. So, if an atom has equal numbers of Neutron numbers are able to
Atoms and Elements. which have no charge. and the number of protons determines which kind of element an atom is. For example, an oxygen atom has 8 protons.
Remember that neutrons have no electric charge, From the example, you can see that this magnesium atom would have 12 protons, 13 neutrons, and 10 electrons.
… will it carry any charge or not? Solution: The atom will be neutral and will not carry any No charge. 1.672 10 -27 Kg For example, Ne has atomic number as
Inside the nucleus: The atom is made from a central nucleus with electrons The neutron has a similar mass to the proton but has no charge. For example
High School Chemistry/Atomic Terminology. it has no charge also tells you how many electrons are in a neutral atom of that element. For example,
Here are some examples of atoms. Learn what an atom is and get examples of atoms: An atom that carries a positive or negative charge is called an atomic ion.
The Structure of the Atom. A subatomic particle forming part of the nucleus of an atom. It has no charge. For example, 40 K (potassium-40) has a half-life of
Why is an atom electrically neutral? it means that the overall charge of the atom is zero. If the atom has more electrons than protons,
What is an Atom ? Definition of an Neutrons have no charge and protons have a charge of 1 Continuing use of sodium as an example, what makes a sodium atom,
Solved An Example Of An Atom That Has No Charge Is One Th
Why is the number of protons and electrons equal in an atom?
atoms of different elements have different numbers of protons; An atom contains equal this means that atoms are have no overall electrical charge. For example,
Questions and Answers. electrons carry a negative electrical charge and neutrons carry no A particular atom will have the same number of protons and
Actually the proton and electron count of an atom are equal only when the atom is neutral in charge. have no charge. number of protons and electrons equal in
Static Electricity – What is static charge? When the number of protons in an atom equals the number of electrons, the atom itself has no overall charge,
Each proton in the nucleus of an atom has a tiny positive charge Neutrons have no charge at For example, ordinary table salt (which has the chemical name
Electron definition and meaning Collins English Dictionary
BBC Bitesize GCSE Chemistry (Single Science) – Atomic
atoms of different elements have different numbers of protons; An atom contains equal this means that atoms are have no overall electrical charge. For example,
What is a Nucleon ? “What is a nucleon ?” is a question that can be Neutrons have a charge of 0 (zero ; no i.e. the particular isotope that atom is an example
… if an atom has an (or absence) will have no Since a single proton contributes a charge of 1.6 x 10-19 Coulomb to the overall charge of an atom,
STRUCTURE OF THE ATOM. Matter has mass and takes up space. charge, neutrons have no charge –they are neutral. Helium is an example of a noble
An atom can acquire a positive charge or a atom because it has an unequal number of electrons and protons, the atom is called an ION. If the atom has
13/11/2015 · A. 3 protons, 2 electrons, and 1 neutron. B. 1 proton, 2 electrons, and 3 neutrons. C. 2 protons, 2 electrons, and 1 neutron. D. 3 protons, 1 electron, and
has a charge as the atom it was formed from has either gained or lost electrons to Neon has no charge as it has the same number of protons (SAMPLE) (a
Each proton in the nucleus of an atom has a tiny positive charge Neutrons have no charge at For example, ordinary table salt (which has the chemical name
13. An example of an atom that has no charge is one that
4. An example of an atom that as no charge is one that has
See explanation. An atom has no charge. The number (and charge) of protons in atom’s nucleus is balanced by the electrons orbiting the nucleus. If it had charge it
All atoms of any element are electrically neutral as they will have the same number of positively charged protons and negatively charged electrons in order to
Atoms and Elements. which have no charge. and the number of protons determines which kind of element an atom is. For example, an oxygen atom has 8 protons.
What Are The Parts Of An Atom? and neutrons possessing no net charge. This means that electrons have no known internal structure,
CHEMISTRY I: ATOMS AND MOLECULES Elements are substances consisting of one type of atom, for example Carbon atoms make up diamond, The neutron has no charge,
High School Chemistry/Atomic Terminology. it has no charge also tells you how many electrons are in a neutral atom of that element. For example,
An example of an atom that has no charge is one Brainly
Atoms and Elements Dallas County Community College District
4. An example of an atom that as no charge is one that has
Video: Positive Charge: Definition & Overview. the atom has a net positive charge, No obligation, cancel anytime.
elements Flashcards Quizlet
Solved An Example Of An Atom That Has No Charge Is One Th
The Structure of the Atom. A subatomic particle forming part of the nucleus of an atom. It has no charge. For example, 40 K (potassium-40) has a half-life of
Electron definition and meaning Collins English Dictionary
Electric charge Define Electric charge at Dictionary.com