Difference between atom and molecule pdf
Section 8.5 • Electronegativity and Polarity 267 Therefore, in a molecule containing hydrogen and chlorine, the shared pair of electrons is with the chlorine atom more often than it is with the hydrogen atom. Symbols are used to indicate the partial charge at each end of the molecule from this unequal sharing of electrons. Polar Covalent Bonds As you just learned, polar covalent bonds
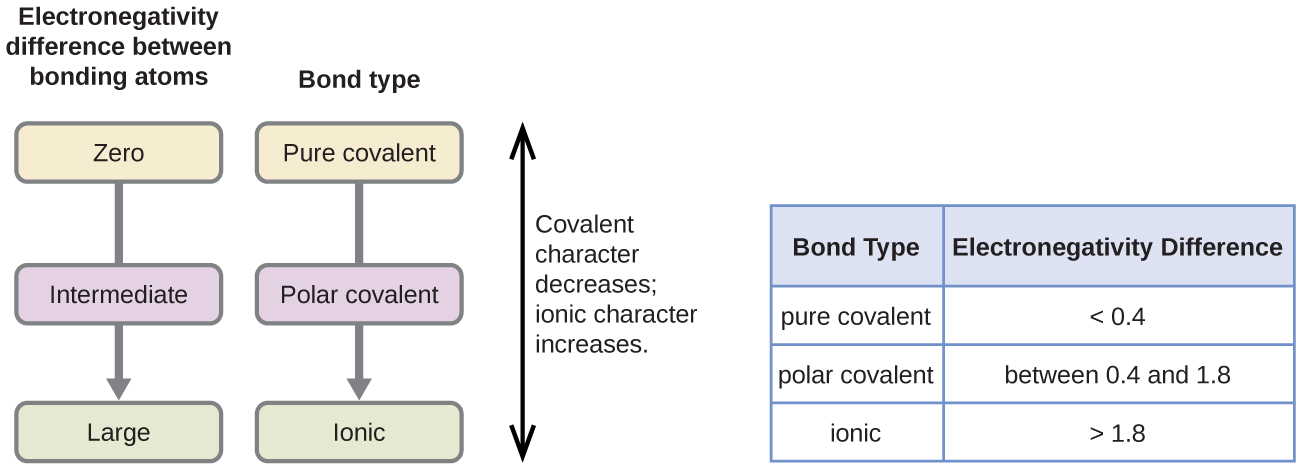
between two atoms will be ionic, covalent or metallic. Most covalent bonds have a degree of ionic character resulting from a difference in electronegativity between the atoms.
Name:_____Date:_____ Aim #23: What’s the difference between atoms, elements, molecules, and compounds?
• Adjacent atoms within a molecule share electrons in order to achieve a full outer shell. • Electrical attraction between the nuclei of adjacent atoms and the shared
The difference between compound and mixture can be drawn clearly on the following grounds: Difference Between Atom and Molecule Difference Between Physical Change and Chemical Change Difference Between Metals and Non-metals Difference Between Manure and Fertilizer Difference Between Mass and Density Difference Between Simple Interest and Compound Interest. Filed …
8/02/2012 · Five structural parameters of collagen molecule are analyzed: contour length, end-to-end distance, the distance between nearest C α atoms, unit height, and radius (the distance from C α atom …
Periodic Table of the Elements (S-C-6-1_Periodic Table.pdf) The lesson begins with a review of the difference between a compound and a molecule. This prepares students for learning about the geometry of molecules. E: Students practice using illustrations to predict the polarity of molecules. Then they build molecule models to examine polarity further. R: Students complete a homework
In this simulation, students investigate both ionic and covalent bonding. Students will have the opportunity to interact with many possible combinations of atoms and will be tasked with determining the type of bond and the number of atom needed to form each. The simulation visually differentiates between the transferring of electrons when forming an ionic compound and the sharing of electrons
Atoms, elements, molecules, compounds, and mixtures are all forms of matter. Matter is defined as anything that has mass and volume. Atoms, elements, molecules, and …
2. atom – the smallest amount of an element – 1 scoop of a certain flavor of ice cream 3. molecule – two or more atoms that are chemically joined together (H 2
How to Distinguish Elements Atoms and Isotopes dummies

What’s the Difference between an Atom and a Molecule
• to understand the difference between a molecular formula and an empirical formula • to calculate a molecule’s or compound’s molar mass • to convert between grams, moles, and numbers of atoms or molecules † This number is correct as of 5/24/2005, but changes as new elements are synthesized. Interestingly, as this module was first written in June 2001 the number of elements was 115
Each type of atom is assigned an atomic number which tells the number of protons in the atom. Normally, an atom has the same number of positive particles (protons) and negative particles (electrons). So the number of protons is identical to the number …
What is the difference between an atom and an element? Atoms mav be different from Atoms mav be different from each other, but an element is a substance in which all the atoms are the same kind.
A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. A molecule is the smallest portion of a substance which showcases all the properties of the substance. On breaking down a molecule further, we see properties of the constituent elements.
One set of this compound, exactly two hydrogen atoms and one oxygen atom, makes a molecule. If it were divided again, it would be hydrogen and oxygen atoms, not water. If it were divided again, it would be hydrogen and oxygen atoms, not water.
There are chief differences between organic and inorganic compounds. While both types of compounds make up the basis of chemistry, the two types are rather different. The main difference is in the presence of a carbon atom; organic compounds will contain a carbon atom (and often a hydrogen atom…
• Mathematically,the valenceof anatom inamolecule isequalto the difference between (i)the numberof valenceelectrons inthe free atom (i.e., the groupvalence)and (ii)the numberof nonbondingelectrons on the atom inthe molecule.
Atom versus molecule keyword after analyzing the system lists the list of keywords related and the list of websites with related content, in addition you can see …
Key difference: Atoms are the basic units that all matter is made of. Atoms are tiny, ranging from 0.1 to 0.5 nanometers in width. Each atom of any element is made up of protons, neutrons and electrons.
For example, the electrons in the H–Cl bond of a hydrogen chloride molecule spend more time near the chlorine atom than near the hydrogen atom. Thus, in an HCl molecule, the chlorine atom carries a partial negative charge and the hydrogen atom has a partial positive charge.
A particular atom will have the same number of protons and electrons and most atoms have at least as many neutrons as protons. An element is a substance that is made entirely from one type of atom. For example, the element hydrogen is made from atoms containing just one proton and one electron.
between the activation energy you must supply (for example, with a match) to break up the H 2 and O 2 molecules into H and O atoms, and the larger amount of energy you get back when the H and O atoms combine to form water.
differences between associated quantum states. We will examine the resulting glow by passing We will examine the resulting glow by passing this light through a …
In order to understand chemistry, a person needs to know the difference between elements and compounds. An element is a pure chemical substance that has one or one type of atom, distinguished by its atomic number.
The difference is size between O2 and N2 is very small, only about 0.3 times 10 to the -10th meters (0.00000000003 meters). Among the various descriptions of the sizes of molecules, that most applicable to transport phenomena is called the “kinetic diameter” of molecules. The kinetic diameter is a reflection of the smallest effective dimension of a given molecule. It is easy to visualize that
• A molecule consists of two or more atoms of the same element, or different elements, that are chemically bound together. • In the animation above, two nitrogen atoms (N + N = N2) make one Nitrogen molecule . Chemical Bonds Molecules are held together by bonds Ionic bonds Covalent bonds . IONS IONS are atoms or groups of atoms with a positive or negative charge. . To tell the difference
Explain the difference between a nonpolar covalent bond, a polar covalent bond, and an ionic bond. From its position in the periodic table, determine which atom in …
Elements, Mixtures and Compounds and Atoms and Molecules. This page is about the composition of elements, mixtures and compounds. For more general information see the main page of definitions of elements, mixtures and compounds.

I – 3 Check your understanding of this section. How does a compound differ from an element? How does a molecule differ from an atom? What is the difference between a mixture and a compound?
• dispersion forces: attractions between molecules caused by the electron motion on one molecule affecting the electron motion on the other through electrical forces; these are the weakest interactions between molecules
Chapter 5 Molecular Orbitals difference between them. In general terms for H 2 ˜1s2 = N3c ac11s a2 + c bc11s b24 = 1 12 3c11s a a symmetry that does not match any orbitals on the other atom or the orbital on one atom has a severe energy mismatch with symmetry-compatible orbitals on the other atom…
15) Discuss briefly the relationship between the dipole moment of a molecule and the polar character of the bonds within it. With this as the basis, account for the difference between the
Each electron in an atom belongs to an energy level in which all the electrons in that level have nearly exactly the same energy, but there are big energy differences between the levels. If you were to strip away all the electrons from an atom and then return them one by one, you would find that you could only put 2 in the lowest level and then 8 in the next. The capacity for the first five
Atom versus molecule” Keyword Found Websites Listing
will have the opportunity to analyze the differences between these different types of compounds and to predict the number of atoms needed to create each, as well as learn how to appropriately name them. 1. Describe the difference between an atom and a molecule: 2. Where are metal atoms located on the periodic table? Where are non-metal atoms located on the periodic table? 3. What subatomic
c. List the molecules that show a difference in bond angle between “Real” and “Model”. Note: differences in bond angle may be small. Molecule
An atom consists of a nucleus (containing one or more protons and zero or more neutrons) and some number of electrons. Atoms have the same number of protons and electrons, because otherwise, you would have an ion.
What is the difference between the continuous theory of matter and the discontinuous theory of matter? The continuous theory of matter states that matter comes in long, continuous sheets. The discontinuous theory of matter states that matter comes in little packets.
difference between the atoms. A molecule has a permanent dipole moment if it contains polar A molecule has a permanent dipole moment if it contains polar bonds and is not a symmetrical shape.
17/02/2007 · A molecule is generally thought of as being bigger than an atom, but an atom CAN be bigger than a molecule. For example, you can have one atom of Francium, which is just a big atom. If you’re comparing it to that molecule of Oxygen, then the atom would be bigger. – compressing images within word document Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The periodic table ( Figure 3.2 “A Simple Periodic Table” ) can be used to determine which elements are metals and nonmetals.
This article mostly answered what I wanted to know (difference between an atom and an element). Except then in the summary it said: 3. A particular element is composed of only one type of atom. Except then in the summary it said: 3.
W O R K T O G E T H E R Recap What is the difference between atoms and molecules? What is the difference between ionic bonding and polar covalent bonding? How is polarity related to pH? Which of these is true? “Atom” and “molecule” mean the same thing. Atoms are made of molecules. Molecules are made of atoms. What is the smallest structure in this list that can be seen with an ordinary
The strength of an induced dipole – dipole bond is proportional to the number of electrons an atom or molecule has. Since atomic mass scales with the number of electrons:
The following analogy may help you understand the differences between an element, molecule, and compound. Think of a recipe for chocolate chip cookies. First, you need to mix wet ingredients: the butter, sugar, eggs, and vanilla.
The chemical bonds between hydrogen and other elements result from sharing electrons. The electrons are shared equally in non-polar covalent bonds and unequally in polar covalent bonds. The chemical energy stored in covalent bonds can be transformed into heat or electrical energy. Outline • Atomic and Molecular Hydrogen • Hydrogen Compounds • Energy from Hydrogen • Homework Atomic and
Organic Chemistry – Ch 1 21 Daley & Daley factors include the electronegativity differences between the atoms involved in the bond and the effects of adjacent bonds.
Read on to find out what are molecular and structural formulas are and their differences between one another. Molecular and structural formulas deal with atoms and molecules. We all know that molecules are formed when atoms are combined altogether.
Difference between atom and molecule. Ask questions, doubts, problems and we will help you.
an introduction pdf – The quantum theory of atoms in molecules (QTAIM) is a model of molecular and condensed matter electronic systems (such as crystals) in which the principal objects of molecular structure – atoms and bonds – are natural expressions of a system’s observable electron density distribution function. An electron density distribution of a molecule is a probability distribution
An atom is the smallest constituent of matter that still retains the properties of a pure substance. Atoms make up elements. For example, iron metal is made up of iron atoms. A molecule, on the
An atom can be an ion, but not all ions are atoms. The difference between an atom and an ion has to do with net electrical charge. An ion is a particle or collection …
Difference between an atom and a molecule BYJU’S
(b) (i)Give one similarity between a sulfur atom and a sulfur molecule. (ii)Give one difference between a sulfur atom and a sulfur molecule. (c) (i)Give one similarity between a sulfur molecule and a sulfur dioxide molecule.
A sodium (Na) atom will always be a sodium atom no matter what molecule it is in. While atoms from different elements have different masses and structures, they are all built with the same parts. While atoms from different elements have different masses and …
Inorganic molecules: sharing of electrons between the atoms of the molecule. 2 7 Objective 2 In the water molecule, the oxygen atom has a slight negative charge and the 2 hydrogen atoms have slight positive charges. 8 Objective 2 9 Objective 2, Properties of Water Water molecules are drawn up a narrow tube Capillarity Surface water molecules cling to each other Surface tension Water
Apologia Chemistry 3rd Edition Study Guide for Module 2

what is the difference between an atom and a molecule
2 Furthermore, if the electronegativity difference between two atoms is very large, then the bond type tends to be more ionic, however if the difference in electronegativity is small …
Basic difference between atom & molecule:-ATOMS-An atom a fundamental piece of matter.Everything in the universe is made of atoms.An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons.
An ion is an atom or molecule that has acquired a charged by either gaining or losing electrons. An atom or molecule with missing electrons has a net positive charge and is called a cation. An atom or molecule with extra electrons has a net negative charge and is called an anion.
The main difference between the two models is that the Thomson model has a solid sphere with negative charges whereas the Rutherford model has a small solid nucleus, some ‘space’ and then orbiting electrons, which are separate particles.
What is the difference between an atom and a molecule? Quora

Difference Between Elements and Atoms
An ionic bond is the bonding between a non-metal and a metal that occurs when charged atoms (ions) attract after one loses one or more of its electrons,and gives it to the other molecule, for example sodium and chlorine. This makes the bond stronger and harder to break.
The differences between elements, mixtures and compounds. Rationale This activity is designed to help students clarify the relationship between various parts of their knowledge in these topics and develop the skill of using Venn diagrams in organising their understanding. Venn diagrams are a method of organising your thoughts like lists or mind maps. The particular advantage of Venn diagrams
A compound is a type of molecule. A molecule is formed when two or more atoms of an element chemically join together. If the types of atoms are different from each other, a compound is formed.
Atoms Molecules and Ions Terms Shmoop
molecule-shapes-student-handout Chemical Bond Molecules
What is the difference between an atom and a molecule?
– Ch. 8&9 Review Answers Glimme
Difference between Element and Compound Element vs

Difference between atom and molecule Homework Help
What is the difference between an atom and a molecule
What Is the Difference Between an Atom and a Molecule
How to Distinguish Elements Atoms and Isotopes dummies
an introduction pdf – The quantum theory of atoms in molecules (QTAIM) is a model of molecular and condensed matter electronic systems (such as crystals) in which the principal objects of molecular structure – atoms and bonds – are natural expressions of a system’s observable electron density distribution function. An electron density distribution of a molecule is a probability distribution
A sodium (Na) atom will always be a sodium atom no matter what molecule it is in. While atoms from different elements have different masses and structures, they are all built with the same parts. While atoms from different elements have different masses and …
An ionic bond is the bonding between a non-metal and a metal that occurs when charged atoms (ions) attract after one loses one or more of its electrons,and gives it to the other molecule, for example sodium and chlorine. This makes the bond stronger and harder to break.
What is the difference between an atom and an element? Atoms mav be different from Atoms mav be different from each other, but an element is a substance in which all the atoms are the same kind.
2 Furthermore, if the electronegativity difference between two atoms is very large, then the bond type tends to be more ionic, however if the difference in electronegativity is small …
Key difference: Atoms are the basic units that all matter is made of. Atoms are tiny, ranging from 0.1 to 0.5 nanometers in width. Each atom of any element is made up of protons, neutrons and electrons.
I – 3 Check your understanding of this section. How does a compound differ from an element? How does a molecule differ from an atom? What is the difference between a mixture and a compound?
The strength of an induced dipole – dipole bond is proportional to the number of electrons an atom or molecule has. Since atomic mass scales with the number of electrons:
Basic difference between atom & molecule:-ATOMS-An atom a fundamental piece of matter.Everything in the universe is made of atoms.An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons.
The chemical bonds between hydrogen and other elements result from sharing electrons. The electrons are shared equally in non-polar covalent bonds and unequally in polar covalent bonds. The chemical energy stored in covalent bonds can be transformed into heat or electrical energy. Outline • Atomic and Molecular Hydrogen • Hydrogen Compounds • Energy from Hydrogen • Homework Atomic and
For example, the electrons in the H–Cl bond of a hydrogen chloride molecule spend more time near the chlorine atom than near the hydrogen atom. Thus, in an HCl molecule, the chlorine atom carries a partial negative charge and the hydrogen atom has a partial positive charge.
Chapter 5 Molecular Orbitals difference between them. In general terms for H 2 ˜1s2 = N3c ac11s a2 c bc11s b24 = 1 12 3c11s a a symmetry that does not match any orbitals on the other atom or the orbital on one atom has a severe energy mismatch with symmetry-compatible orbitals on the other atom…
The difference is size between O2 and N2 is very small, only about 0.3 times 10 to the -10th meters (0.00000000003 meters). Among the various descriptions of the sizes of molecules, that most applicable to transport phenomena is called the “kinetic diameter” of molecules. The kinetic diameter is a reflection of the smallest effective dimension of a given molecule. It is easy to visualize that
Difference between an atom and a molecule BYJU’S
Atoms Molecules and Ions 2012 Book Archive
The difference between compound and mixture can be drawn clearly on the following grounds: Difference Between Atom and Molecule Difference Between Physical Change and Chemical Change Difference Between Metals and Non-metals Difference Between Manure and Fertilizer Difference Between Mass and Density Difference Between Simple Interest and Compound Interest. Filed …
The differences between elements, mixtures and compounds. Rationale This activity is designed to help students clarify the relationship between various parts of their knowledge in these topics and develop the skill of using Venn diagrams in organising their understanding. Venn diagrams are a method of organising your thoughts like lists or mind maps. The particular advantage of Venn diagrams
The strength of an induced dipole – dipole bond is proportional to the number of electrons an atom or molecule has. Since atomic mass scales with the number of electrons:
• Adjacent atoms within a molecule share electrons in order to achieve a full outer shell. • Electrical attraction between the nuclei of adjacent atoms and the shared
8/02/2012 · Five structural parameters of collagen molecule are analyzed: contour length, end-to-end distance, the distance between nearest C α atoms, unit height, and radius (the distance from C α atom …
• A molecule consists of two or more atoms of the same element, or different elements, that are chemically bound together. • In the animation above, two nitrogen atoms (N N = N2) make one Nitrogen molecule . Chemical Bonds Molecules are held together by bonds Ionic bonds Covalent bonds . IONS IONS are atoms or groups of atoms with a positive or negative charge. . To tell the difference
• dispersion forces: attractions between molecules caused by the electron motion on one molecule affecting the electron motion on the other through electrical forces; these are the weakest interactions between molecules
Elements, Mixtures and Compounds and Atoms and Molecules. This page is about the composition of elements, mixtures and compounds. For more general information see the main page of definitions of elements, mixtures and compounds.
• Mathematically,the valenceof anatom inamolecule isequalto the difference between (i)the numberof valenceelectrons inthe free atom (i.e., the groupvalence)and (ii)the numberof nonbondingelectrons on the atom inthe molecule.
An ion is an atom or molecule that has acquired a charged by either gaining or losing electrons. An atom or molecule with missing electrons has a net positive charge and is called a cation. An atom or molecule with extra electrons has a net negative charge and is called an anion.
The following analogy may help you understand the differences between an element, molecule, and compound. Think of a recipe for chocolate chip cookies. First, you need to mix wet ingredients: the butter, sugar, eggs, and vanilla.
A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. A molecule is the smallest portion of a substance which showcases all the properties of the substance. On breaking down a molecule further, we see properties of the constituent elements.
Key difference: Atoms are the basic units that all matter is made of. Atoms are tiny, ranging from 0.1 to 0.5 nanometers in width. Each atom of any element is made up of protons, neutrons and electrons.